5581
Peripheral Nerve Stimulation in MRI: Insights from a three level analysis and coupled EM-electrophysiological simulations in neuro-functionalized human models1IT'IS Foundation, Zurich, Switzerland, 2Max Plank Institute - Tuebingen, 3Zurich MedTech, 4Max Plank Institute - Tuebingen, Germany, 5ETH, Zurich, CH
Synopsis
The mechanisms of peripheral nerve stimulation (PNS) induced by the fast switching of MRI gradient coils are only partially understood, stimulation sites and E-field (or dB/dt) thresholds show large inter-subject variability and neurostimulation models based on the amphibian SENN axon model are not ideal. We propose a 3 level computational investigation that combines analysis of E-field exposure, of activation functions, and of multi-parametric EM-electrophysiological simulations in neuro-functionalized human models for different axon models and gradient waveforms. Results concerning E-field/dB/dt thresholds values and sites of neurostimulation are compared with published experimental data. A functional uncertainty analysis is also provided.
Introduction
Limitations of MRI gradient outputs (dB/dt and/or E-field) are imposed by the IEC-60601-2-331 standard according to the IEEE2 and ICNIRP3 guidelines to avoid peripheral nerve stimulation (PNS) on the basis of experimental evidence4 and strength-duration (SD) relations derived from neurostimulation models using the compartmentalized axonal SENN5 model. Many reasons exist for further investigations6,7. Some authors8 suggest that higher threshold levels (i.e. 120%) than those indicated in IEC-60601-2-331 can be used in clinical practice. The mechanisms of MRI gradient induced PNS are still only partially understood7, the sites and thresholds of neurostimulation depend on MRI pulse sequence parameters, and they show large inter-subject variability8,9. Additionally, it has become clear that SENN may not be ideal and a conservative model for mammalian axons at 37C6. In the present work we analyse PNS induced by MRI gradients in human bodies using a 3 level approach comparing whole body, MRI pulse sequence-independent E-field exposure analysis (i) with neuronal activation functions (ii) and coupled EM-electrophysiological simulations in neuro-functionalized human body models, featuring different axonal models and considering realistic clinical gradient pulse sequences (iii). Benefits and limitations of the approaches are discussed, conditions and mechanisms for the stimulation investigated, and results are compared with experiments in terms of the location of neurostimulation and thresholds.Methods
Electromagnetic and neuronal
simulations were performed using Sim4Life. 1th level: the E-fields and
their Jacobian (as a measure for inhomogeneity – a relevant stimulation
mechanism not adequately considered by current standards) were computed for
each individual gradient coil within the “Ella” and “Fat” human models from the
Virtual Population10 at three relevant body positions (head, hearth
and pelvis at coil’s isocentre). 2nd level: Large sets of generic,
approximated nerve trajectories were generated in the arms, legs and spinal
cord. E-fields and activation functions11 were calculated along these
trajectories. 3rd level: Anatomically realistic nerve trajectories
were parametrized as dynamic 15um diameter axon-models (amphibian SENN5,
warm (37deg) mammalian-specific McIntyre12 and Sweeney13 models).
E-fields and neuronal simulations were coupled using the ‘extracellular
mechanism’ available in the NEURON libraries integrated in Sim4Life. Biphasic
E-field pulse waveforms, derived from trapezoidal gradient pulse shape typical
of 2D and 3D EPI imaging, as well as transient exposure derived from clinically-employed
2D and 3D spiral pulses, were investigated. Threshold fields and the location
of spike initiation were recorded for each configuration.
Results
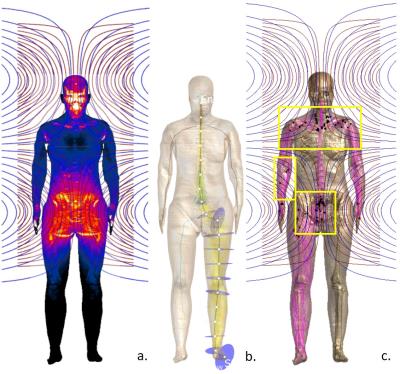
Locations of neurostimulation derived from the 3rd-level approach were compared to results from the other 2 levels (E-field maps and activation functions) for trapezoidal, distinct (i.e., individual gradient coils) and simultaneous gradient pulse sequences. The loci of non-end node activations (see figure 1c) predicted by the electophysiological models are in general agreement with experimental findings, such as in Hoffmann7, where activation is detected in the triceps muscles in the upper arm and scapula. For the same MR sequence, the exact stimulation locations depended on which of the three axonal models is used, but are generally located near articulations or at muscle/fat interfaces. Threshold predictions showed large model-dependence. Location of likeliest excitation can change when going from single gradient-coil exposure to pulse sequences involving simultaneous coil-activation.Discussion
The 3rd level approach permits to relate the location of stimulation to anatomical factors (dielectric contrast at tissue interfaces, field inhomogeneity and foci near articulations), and to consider realistic nerve trajectories and pulse sequence timing. The expected observence of stimulation at many end-nodes suggests that additional studies are required to improve models for nerve terminations. Results must be revised with more comprehensive and (anatomically and electrophysiologically) accurate nerve trajectory functionalization. The agreement with experimentally verified neurostimulation sites and levels is promising.Conclusion
The results show that the proposed 3rd level analysis permits to investigate different mechanisms of MRI induced neurostimulation, with increasing consideration for anatomical, electrophysiological, and pulse-sequence specific information that increase safety and potentially reduce the need for conservative safety margins. Results could impact existing safety guidelines and the optimization of MRI pulse sequences. Extensive verification has been performed and validation is ongoingAcknowledgements
No acknowledgement found.References
1. IEC 60601-2-33:2010. Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis.
2. IEEE Std C95.6:2002. IEEE Standard for safety levels with respect to human exposure to electromagnetic fields, 0-3kHz
3. ICNIRP Guidelines for limiting exposure to time-varying electric and magnetic fields (1Hz-100kHz). 2010. Health Physics 99(6): 818-836
4. Bourland et al. “Physiologic effects of intense MR imaging gradient fields”. Neuroim Clin Am. 1999 9: 363-377
5. Reilly P. et al. ‘Sensory effects of transient electrical stimulation: evaluation with a neuroelectrical model, IEEE Trans.BME. 1985. 32: 1001–1011
6. Reilly J. et al. “Low-frequency electrical dosimetry: research agenda of the IEEE International Committee on Electromagnetic Safety." Physics in Medicine and Biology. 2016. 61 (12): 138.
7.Neufeld E. et al. “Functionalized anatomical models for EM-neuron interaction modelling”, Phys. Med. Biol. 2016, 61(12): 4390
8. Vogt et al. “Increased time rate of change of gradient fields: effect on peripheral nerve stimulation at clinical MRI Imaging”. Radiology. 2004. 233(2): 548-554
9. Hoffmann et al. “Electromyography in MRI – first recordings of peripheral nerve activation caused by fast magnetic field gradients”. Magnetic Resonance in Medicine. 2000. 43: 534-539
10. Christ A. et al. ”The Virtual Family - development of surface-based anatomical models of two adults and two children for dosimetric simulations”, Physics in Medicine and Biology, 2010. 55(2): 23-38
11. Rattay, F. "The basic mechanism for the electrical stimulation of the nervous system." Neuroscience. 1999. 89(2): 335-346.
12. McIntyre C.C. et al. “Modelling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle”. J Neurophysiol. 2002: 87(2):995-1006.
13. Sweeney J.D. et al. “Modelling of mammalian myelinated nerve for functional neuromuscular stimulation”. IEEE/9th Annual Conference of the Engineering in Medicine and Biology Society. 1986. 1577-1578