5577
RF safety assessment of a 32-channel integrated body coil for 7 Tesla: Thermal dose evaluation at high SAR level1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Erwin L. Hahn Institute for MRI, University Duisburg-Essen, Essen, Germany, 3High Field and Hybrid MR Imaging, University Hospital Essen, Essen, Germany
Synopsis
RF safety assessment for a 32-ch body coil for 7T was performed based on SAR, tissue temperature, and a thermal dose model (CEM43°C). Temperature simulations considered a temperature-dependent thermoregulation. The tissue temperature limit is exceeded when SAR limits are adhered to. However, based on the thermal dose limit, the maximum input power determined from SAR limits can be exceeded by up to a factor of 5 without noticeable limitations in permissible exposure time in MR examinations. This increased input power allows for improved B1+ homogeneity with 50% reduced flip angle error compared to the input power determined from SAR limits.
Introduction
PTx systems with multi‐channel transmit arrays provide high degrees of freedom for the manipulation of RF fields. RF coil arrays placed behind the bore liner allow for a higher number of coil elements and provide superior patient comfort. RF safety assessment requires simulations with heterogeneous body models. However, limits are defined for both SAR and tissue temperature, and the spatial distributions of both do not correlate well in all cases. Thermal dose potentially allows a more precise determination of the subject’s RF exposure, and the evaluation of thermal dose is under consideration for future regulatory limits. Thermal dose limits could allow for longer exposure times and/or higher SAR, and the latter can result in improved RF shimming performance. In this study, an RF exposure assessment was performed for a 32‐ch whole‐body coil array configuration 1 based on SAR, local tissue temperature, and thermal dose CEM43°C 2,3.Methods
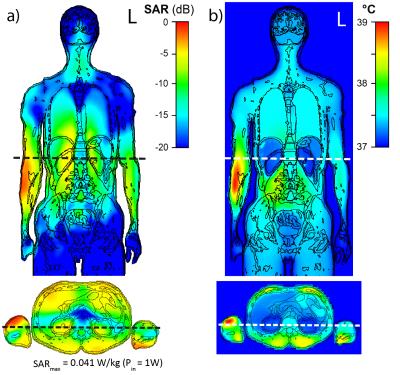
A 32-ch Tx/Rx meander body array was placed behind the bore liner and loaded with a heterogeneous body model (72.4 kg) in head-first supine position with the liver-kidney region in the coil center, Fig. 1. RF shimming regarding uniform B1+ in the central axial slice was performed for a target magnetization of 6.5 µT and maximum peak power per channel of 500 W. For SAR evaluation, an MR protocol with duty cycle of 10% (fast pulses) 4 was assumed. Thermal simulations were performed using the Pennes bioheat equation 5 with the following thermal parameters: ambient temperature = 25 °C, blood temperature = 37 °C, heat convection coefficient at body surface = 2 W/(m²∙K). A temperature-dependent physiological response to RF exposure with exponential increase in blood perfusion was applied 6 as proposed for healthy subjects. Thermal results were evaluated regarding tissue temperature and exposure time at thermal dose limit of CEM43°C = 9 min for healthy subjects 3. Thermal simulations were performed for an input power (Pin) determined based on the SAR10g limit for normal operating mode (NM) and for Pin increased by a factor of 4 and 5. Corresponding permissible input power and exposure times and consequences for RF shimming were determined. Simulations were performed in CST Studio Suite 2016 7.Results and discussion
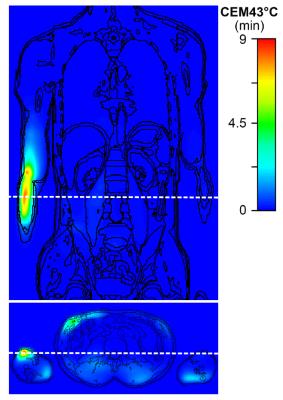
An RF shim setting with mean deviation from target magnetization of 30% and SAR10g,max of 20 W/kg (SAR limit in the limbs in NM) was selected, Fig. 2. SAR10g,max was located close to the elbow joint in the right arm, cf. Fig. 3 a). The maximum permissible time‐averaged input power determined from the SAR limit was 482 W and the corresponding maximum tissue temperature of 39.24 °C was found close to the location of the SAR10g,max, Fig. 3 b). As a result, the local temperature limit of 39 °C in normal mode (NM) was exceeded even though the SAR limits were complied with; to satisfy the temperature limit, the maximum permissible input power would have to be decreased. The temperature limit was reached after 10 min exposure time. However, the thermal dose limit of CEM43°C = 9 min allowed an exposure time > 30 h. The permissible exposure time decreases to 1.4 h and 50 min with Pin increased by a factor of 4 and 5, respectively, cf. Table 1. Thus, the CEM43°C approach allows for continuation of the MR examination after the time point when the temperature limit in NM is reached. With an exposure time of 1.4 h, most MR examinations will be feasible without noticeable limitations. Furthermore, the temperature as well as the thermal dose maximum were located in well-perfused muscle tissue for all considered power levels, Fig. 4. In the literature, minor thermal damage in muscle was found above CEM43°C = 41 min and thermal thresholds for different tissue types are discussed 3. Tissue-dependent thresholds would allow for even longer exposure times; however, the position of the temperature maximum must be known. With Pin increased by a factor of 4 and 5, the B1+ homogeneity can be improved and the flip angle (FA) error decreased to 17.46% and 15.91%, respectively, cf. Fig. 2.Conclusion
The proposed perfusion model yields a tissue temperature that exceeds the temperature limit even when SAR limits are adhered to. The maximum permitted input power would need to be decreased if temperature is considered to be the fundamental limit most closely related to possible tissue damage. Compared to the temperature limit, the thermal dose approach allows longer exposure time and higher input power. The higher permissible input power can allow for increased shim performance with reduced FA error.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement n. 291903 MRexcite.References
1. Orzada et al. Proc. ISMRM 24 (2016) #0167.
2. Sapareto, Dewey. Int. J. Radiation Oncology Biol. Phys. Vol. 10, pp. 787-800 (1983).
3. Van Rhoon et al. Eur Radiol. 23:2215-2227 (2013).
4. Pennes, H. H. Journal of Applied Physiology Volume 1 (2) (1948).
5. Laakso et al. Phys Med Biol 56, 7449-7471 (2011).
6. CST AG, Darmstadt, Germany.
Figures