5575
Impact of Abdominal Magnetic Resonance Imaging on DNA Double Strand-Breaks in Human Blood Lymphocytes1Department of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany, 2Institute of Medical Radiation Biology, University Hospital Essen, Essen, Germany, 3Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Hospital Essen, Essen, Germany, 4High Field and Hybrid MR Imaging, University Hospital Essen, Essen, Germany
Synopsis
Magnetic resonance imaging is considered to be a safe alternative to other imaging techniques that use ionizing radiation, such as computed tomography. Initially driven by X-ray and CT imaging studies, within the recent years different in vitro and in vivo studies analyzed the impact of MR imaging on DNA integrity in human lymphocytes but reported contradictory results. In this study on patients referred to clinical abdominal MRI, γ-H2AX immunofluorescence microscopy was used to determine DNA integrity. No evidence of DNA damage induced by abdominal MRI in a clinical setting was found.
Purpose
Magnetic resonance imaging (MRI) is a widely used and well-established non-invasive medical diagnostic imaging modality for abdominal imaging. It is considered to be a safe alternative to other imaging techniques that use ionizing radiation, such as computed tomography (CT). Only minor symptoms and transient effects as dizziness or nausea caused by the static magnetic field and reversible heart rhythm disturbances caused by the gradient fields can occur rarely and are not a serious risk to the patient1. Initially driven by studies that reported increased numbers of DNA double-strand breaks (DSB) following X-ray and CT imaging examinations, Fiechter et al. showed an increase of DSB after cardiac MRI by using the γ-H2AX immunofluorescence microscopy2. Following studies could refute these findings3 so that there is a need for further research.
As cardiac exams make up only a small part of MRI in clinical routine, the aim of our study was to analyze DNA DSB in human blood lymphocytes before and after abdominal MRI.
Methods
Patients and MRI protocols
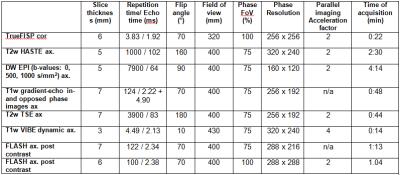
In our preliminary studies, blood samples (10 ml) from currently 8 out of 40 planned patients (six male and two female, mean age: 56.6 years) were obtained before and after contrast agent-enhanced abdominal MRI. All patients were imaged with a 1.5 T MR system (MAGNETOM Aera, Siemens, Healthcare, Erlangen, Germany) equipped with a maximum gradient amplitude of 45 mT/m and a maximum slew rate of 200 (T∙m-1)/sec. The MRI protocol included the following pulse sequences: (a) a coronal true fast imaging with steady-state precession sequence (TrueFISP), (b) an axial T2-weighted single-shot fast spin echo (HASTE) sequence, (c) an axial diffusion weighted echo planar sequence (EPI DWI) with B values of 0, 500 and 1000 s/mm², (d) the acquisition of axial T1-weighted gradient-echo in- and opposed-phase images, (e) an axial T2-weighted fat saturated turbo spin echo (TSE) sequence in breath hold, (f) a dynamical axial T1-weighted VIBE. Four repetitive scans were performed (pre-contrast, arterial phase [20s delay], portal venous phase [60s delay], venous phase [100s delay] after i.v.-injection of 0.1 ml/kg body weight Gadubutrol (Gadovist, Bayer Healthcare, Berlin, Germany), (g) an axial post-contrast T1-weighted Fast Low Angle Shot (FLASH) and (h) a coronal post-contrast T1-weighted FLASH (sequence parameters are given in figure 3).
γ-H2AX immunofluorescence microscopy
DSBs were detected by immunofluorescence microscopy using the accumulation of phosphorylated H2AX at the DSBs. For this experiments rabbit-anti-γ-H2AX antibody was used in combination of a secondary goat-anti-rabbit antibody conjugated with AlexaFluor-488. DNA in the cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). For visualization of the γ-H2AX foci per lymphocyte a fully automated digital microscopy system (MetaSystem instruments) was used, which detected the foci in ~5000 cells per sample. Altogether, 80.000 cells were analyzed.
Statistics
Statistical analysis was performed using MedCalc Version 13.3 (MedCalc Software, Mariakerke, Belgium). A D’Agostino-Pearson test was performed to test for normal distribution. A paired t test was performed to compare the excess foci before MR imaging to the excess foci after MR imaging. A P value of less than 0.05 indicated statistical significance.
Results
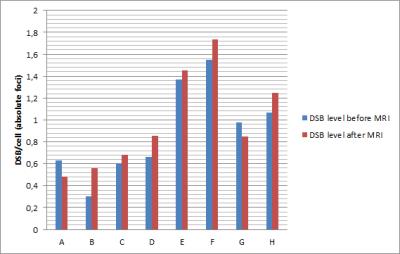
The mean baseline DSB level for all 8 patients before MR imaging was 0.896 ± 0.423 DSB per cell. After MR imaging, a mean DSB level of 0.982 ± 0.450 DSB per cell was determined (Fig. 1). The mean excess foci were 0.086 ± 0.152 DSB per cell. By using a paired t test, no statistically significant differences were found for DSBs induced by MR imaging (P = 0.155).Discussion
Our study demonstrate that there is no significant change in DNA DSBs formation after abdominal MRI compared with the baseline DSBs level obtained before MR imaging. Within the past years, different in vitro and in vivo studies analyzed the impact of MR imaging on DNA integrity in human lymphocytes but reported contradictory results. Some observed an increase in comet formation by performing alkaline single-cell gel electrophoresis, an enhanced number of micronuclei or an increase in DNA DSBs detected with γ-H2AX analysis2, 4, 5. In contrast, different studies did not found such effects induced by MRI3, 6, 7 or observed an enhanced DNA damage only when a contrast agent was administered8. In our in vivo study we investigated abdominal MRI in a clinical setting with contrast agent. Despite the small sample size, our results are contrary to those of reports describing a potential carcinogenic effect of MRI and thus strengthen the acceptance of MRI as a safe imaging method.
Conclusion
By using γ-H2AX immunofluorescence microscopy, we were not able to find any evidence of DNA damage induced by abdominal MRI in a clinical setting.Acknowledgements
No acknowledgement found.References
1 Franco G, Perduri R, Murolo A. [Health effects of occupational exposure to static magnetic fields used in magnetic resonance imaging: a review]. Med Lav 2008;99(1):16-28.
2 Fiechter M, Stehli J, Fuchs TA, et al. Impact of cardiac magnetic resonance imaging on human lymphocyte DNA integrity. Eur Heart J 2013;34(30):2340-5.
3 Brand M, Ellmann S, Sommer M, et al. Influence of Cardiac MR Imaging on DNA Double-Strand Breaks in Human Blood Lymphocytes. Radiology 2015;277(2):406-12.
4 Simi S, Ballardin M, Casella M, et al. Is the genotoxic effect of magnetic resonance negligible? Low persistence of micronucleus frequency in lymphocytes of individuals after cardiac scan. Mutat Res 2008;645(1-2):39-43.
5 Lee JW, Kim MS, Kim YJ, et al. Genotoxic effects of 3 T magnetic resonance imaging in cultured human lymphocytes. Bioelectromagnetics 2011;32(7):535-42.
6 Reddig A, Fatahi M, Roggenbuck D, et al. Impact of in Vivo High-Field-Strength and Ultra-High-Field-Strength MR Imaging on DNA Double-Strand-Break Formation in Human Lymphocytes. Radiology 2016:160794.
7 Reddig A, Fatahi M, Friebe B, et al. Analysis of DNA Double-Strand Breaks and Cytotoxicity after 7 Tesla Magnetic Resonance Imaging of Isolated Human Lymphocytes. PLoS One 2015;10(7):e0132702.
8 Yildiz S, Cece H, Kaya I, et al. Impact of contrast enhanced MRI on lymphocyte DNA damage and serum visfatin level. Clin Biochem 2011;44(12):975-9.
Figures