5570
Immediate Reactions to Gadolinium Based Contrast Agents: a Meta-Analysis1Radiology, Weill Cornell Medical College, New York, NY, United States, 2Department of Healthcare Policy and Research, Weill Cornell Medical College, New York, 3Radiology, Weill Cornell Medical Center, 4Department of Radiology, Cornell and Columbia Universities, New York, NY, United States
Synopsis
Recently there has been increased attention focused on GBCA safety and data on the numbers of reactions have begun to be reported in large Studies. Here we combine data from multiple papers in a meta-analysis to determine if certain GBCA or classes of GBCA may have different immediate reaction profiles.
Purpose
The extraordinarily low incidence of adverse reactions to gadolinium based contrast agents (GBCA) is widely known and makes comparing their relative safety difficult since hundreds of thousands of administrations are necessary to detect differences. Recently there has been increased attention focused on GBCA safety and data on the numbers of reactions have begun to be reported in large case series. Here we combine data from multiple papers in a meta-analysis to determine if certain GBCA or classes of GBCA may have different immediate reaction profiles.Methods
Two authors independently searched the Medline and Google Scholar databases from inception to September 2016 using the following key words: GBCA, gadolinium, contrast media, contrast agent, magnetic resonance imaging, MRI, allergy, acute adverse effect, hypersensitivity, reaction to identify papers reporting on immediate GBCA adverse events . For all papers, we reviewed the titles and abstracts excluding pharmaco-vigilance studies, studies that didn’t use The American College of Radiology (ACR) classification of hypersensitivity reactions, studies with incomplete data, and non-original research (e.g. duplicate studies) and studies that did not evaluate immediate adverse events in a defined population, e.g. case reports. Two authors independently read each of the ten papers meeting inclusion/exclusion criteria, extracting the number of administrations, the number of mild, moderate and severe reactions and the number of deaths for each GBCA. In addition as well as data on gender, age and risk factors was collected. For all meta-analyses, we performed heterogeneity assessment using both Cochran Q statistic and I2 statistics. Random effects were added when severe heterogeneity was detected. The significance of differences in immediate reaction rates as well as severe/fatal reaction rates was calculated and compared for classes of chelate structure and for individual chelates. We considered a type-I error as 0.05 to determine the statistical significance (P-value<0.05). We also conducted additional meta-analyses by comparing overall reaction rates between different genders, and between gadodiamide and each of to the other agents.Results
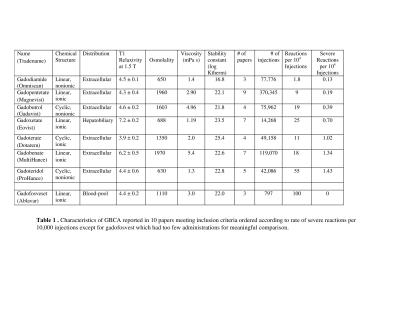
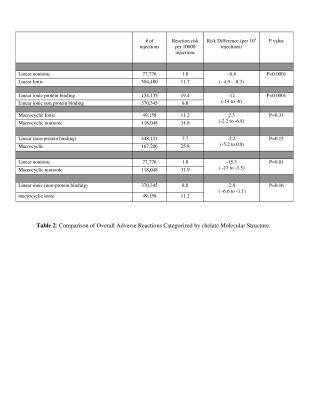
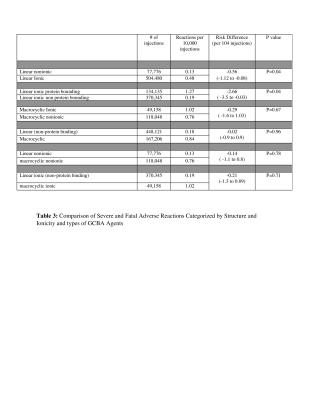
Ten papers met the inclusion criteria covering a total of 749,647 GBCA administrations (1-10) with 1,034 administrations reported to have immediate adverse reactions. The overall rate of patients having immediate reactions was 14/10,000 administrations and the overall rate of severe reactions was 0.52/10,000 with a breakdown by chemical structure in Tables 1-2. In this cohort, 87 % (902/1034) were mild, 9% (93/1034) were moderate and 4% (39/1034) were severe reactions based upon the ACR classification. For 6 papers with data on gender in 640703 patients, there were more adverse reactions reported in female patients, 670 (66%) compared to 347 (34%) in male patients. The pooled risk difference between reaction rates for male versus female was -0.001126 (95% CI = -0.001782 to -0.00047, p = 0.0008). The nonionic linear GBCA, gadodiamide, had the lowest overall rate of immediate adverse reactions (1.8/10000) which was lower than linear ionic (12/10000, p<0.0001) with a pooled risk difference = -6.6 (CI95% -4.9 to -8.3) and lower than nonionic macrocyclic GBCA (32/10000, p=0.01) with a pooled risk difference: -15.3 (CI95% -27 to -3.5). Nonionic linear GBCA also had the lowest rate of severe/fatal reactions (0.13/10,000) compared to linear ionic ( 0.48/10,000), pooled risk difference: -0 .56(CI95% -1.12 to -0.08 )(P=0.04), nonionic macrocyclic GBCA 0.76/10000, -0 .14 (CI95% -1.12 to -0.08 )(P=0.78). Comparing gadodiamide to each of the other GBCA individually, based upon pooled risk differences, gadodiamide had fewer reactions than gadopentetate dimeglumine (p<0.0001), gadobenate (p<0.0001), gadoterate (p=0.0001), gadobutrol (p<0.0001) and gadoteridol (p=0.0009). A comparison to gadofosveset and gadoxetate could not be performed due to the lack of papers utilizing gadodiamide with those agents. Although gadofosveset, had the highest overall rate of reactions (including mild moderate and severe) 100/10000, gadoteridol (1.43/10,000) and gadobenate(1.34/10,000) had the highest rates of severe adverse reactions.Discussion
The extraordinarily low rate of immediate reactions to GBCA and extremely rare incidence of fatal reactions has led to a generalization that GBCA are very safe, especially compared to iodinated contrast media. Differences in reaction rates between GBCA have been felt to be negligible. However, these data compiled from 10 large, well designed retrospective studies of prospectively acquired data including 749,647 gadolinium administrations indicate a 50-fold difference between the agent with the least (gadodiamide) and the most (gadofosveset) immediate reactions. These data further show a higher rate of immediate reactions associated with the properties of protein binding, ionicity and cyclic structure.Conclusion
Combining data from 10 large retrospective reviews in a meta-analysis gives a strong signal that the properties of ionicity, protein binding and macrocyclic structure increase the rate of immediate reactions to GBCA.Acknowledgements
No acknowledgement found.References
1-Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011 Feb;196(2):W138-43.
2- Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012 Aug;264(2):414-22.
3- Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol. 2010 Feb;194(2):430-4.
4- Morgan DE, Spann JS, Lockhart ME, Winningham B, Bolus DN. Assessment of adverse reaction rates during gadoteridol-enhanced MR imaging in 28,078 patients. Radiology. 2011 Apr;259(1):109-16.
5- Davenport MS, Dillman JR, Cohan RH, Hussain HK, Khalatbari S, McHugh JB, Ellis JH. Effect of abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine on rate of allergic-like reactions. Radiology. 2013 Mar;266(3):773-82.
6- Bruder O, Schneider S, Pilz G, van Rossum AC, Schwitter J, Nothnagel D, Lombardi M, Buss S, Wagner A, Petersen S, Greulich S, Jensen C, Nagel E, Sechtem U, Mahrholdt H. Update on Acute Adverse Reactions to Gadolinium based Contrast Agents in Cardiovascular MR. Large Multi-National and Multi-Ethnical Population Experience With 37788 Patients From the EuroCMR Registry. J Cardiovasc Magn Reson. 2015 Jul 14;17:58.
7- Okigawa T, Utsunomiya D, Tajiri S, Okumura S, Sasao A, Wada H, Oda S, Arimura H, Hayashida E, Urata J, Yamashita Y.Incidence and severity of acute adverse reactions to four different gadolinium-based MR contrast agents. Magn Reson Med Sci. 2014;13(1):1-6.
8-Aran S, Shaqdan KW, Abujudeh HH. Adverse allergic reactions to linear ionic gadolinium-based contrast agents: experience with 194, 400 injections. Clin Radiol. 2015 May;70(5):466-75
9- Power S, Talbot N, Kucharczyk W, Mandell DM.Allergic-like Reactions to the MR Imaging Contrast Agent Gadobutrol: A Prospective Study of 32 991 Consecutive Injections. Radiology. 2016 Oct;281(1):72-7.
10- Granata V, Cascella M, Fusco R, dell'Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo A, Petrillo A. Immediate Adverse Reactions to Gadolinium-Based MR Contrast Media: A Retrospective Analysis on 10,608 Examinations.Biomed Res Int. 2016;2016:3918292.