5568
A Manganese-Based Alternative to Gadolinium: Contrast Enhanced MR Angiography at 3T and In Vivo Stability1A. A. Martinos Center for Biomedical Imaging, MGH/ Harvard Medical School, Charlestown, MA, United States
Synopsis
We evaluated the efficacy of a new manganese-based contrast agent, Mn-PyC3A, in contrast-enhanced MR angiography by comparison to Gd-DTPA in a baboon model at 3T. Mn-PyC3A clearance was assessed by dynamically scanning the excretory organs and performing serial blood draws out to 60 min. Mn-PyC3A plasma clearance and metabolism were quantified from the drawn blood. Mn-PyC3A generates equivalent vessel-to-muscle contrast-to-noise ratios as Gd-DTPA at 3T, clears via a mixed renal and hepatobiliary pathway, and is excreted unchanged. Mn-PyC3A is a functionally equivalent gadolinium-free alternative for contrast-enhanced MRI angiography.
Purpose
There is increasing concern about the use and safety of gadolinium (Gd) based contrast agents (GBCAs). Gadolinium exposure is directly linked to nephrogenic system fibrosis in renally impaired patients,1, 2 and these patients are often denied valuable imaging. Long-term gadolinium retention was recently identified in the CNS of patients with normal renal function receiving GBCAs.3, 4 Manganese (Mn) based contrast agents could provide a promising and safer alternative to GBCAs – divalent Mn generates contrast with comparable potency to Gd and Mn is a biogenic element that is readily excreted. However, the Mn ion tends to be kinetically labile with respect to Mn dissociation (ie. the agents fall apart in vivo) and for this reason development of Mn based contrast agents is largely underexplored. We developed Mn-PyC3A as a kinetically inert and high-relaxivity Gd-free alternative to GBCAs.5 The purpose of this study is to assess the efficacy of Mn-PyC3A for MR angiography and to evaluate Mn-PyC3A clearance and metabolism.Methods
Mn-PyC3A is a Mn-based contrast we developed as a GBCA alternative.5 Mn-PyC3A possesses relaxivity equivalent to GBCA such as Gd-DTPA (T1-relaxivity of Mn-PyC3A and Gd-DTPA are 3.6 mM-1s-1 and 3.8 mM-1s-1, respectively, in human blood plasma at 1.4T, 37 °C). Contrast enhanced MR arteriograms were acquired at 3T using 0.1 mmol/kg Mn-PyC3A or 0.1 mmol/kg Gd-DTPA in Papio anubis baboons, N=4 per agent. Animals were imaged using a coronal T1w VIBE sequence with a field of view covering the thorax and abdomen, and imaged prior to and 0.15 min following i.v. injection of the contrast agent. Imaging was repeated out to 60 min in order to evaluate distribution and clearance of the contrast agents. Blood was sampled serially out to 60 min and analyzed using high-pressure liquid chromatography - inductively coupled mass spectrometry (HPLC-ICP-MS) to quantify plasma clearance and to identify any metabolites. Urine was also collected and analyzed for manganese-containing metabolites using HPLC-ICP-MS. Mn-PyC3A vs. Gd-DTPA abdominal aorta vs. psoas muscle contrast-to-noise ratios (CNR) were compared using a paired t-test. The Mn-PyC3A excretion path was assessed by analyzing signal-to-noise ratios normalized to the pre-contrast baseline scan (nSNR) in the kidney and liver as a function of time. Mn-PyC3A and Gd-DTPA plasma clearance rates were estimated from a bi-exponential model of the Mn or Gd plasma data.Results
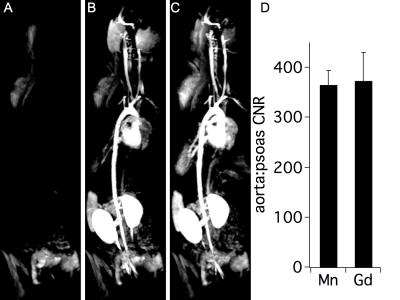
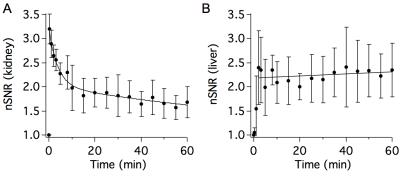
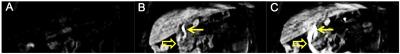
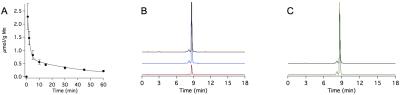
Mn-PyC3A is functionally equivalent to Gd-DTPA in generating contrast for MR angiography at 3T. Figs 1A-C show coronal maximum intensity projections prior to and 0.15 min following injection of Mn-PyC3A and Gd-DTPA, respectively. Quantification of abdominal aorta to psoas muscle CNR for Mn-PyC3A and Gd-DPTA demonstrates equivalence between the agents, CNR = 365±28 after Mn-PyC3A injection vs. 372±56 after Gd-DTPA injection, P = NS. Dynamic imaging of the abdomen reveals mixed renal and hepatobiliary clearance of Mn-PyC3A. Figure 2 depicts nSNR in the kidney and liver as a function of time after Mn-PyC3A injection. Hepatobiliary clearance is confirmed by strong enhancement of the biliary tree beginning at ~20 min. Figure 3 shows a coronal view of the liver and hepatobiliary tree prior to, 20 and 40 min after Mn-PyC3A injection. The common bile duct and duodenum begin to enhance at 20 min and are strongly enhanced at 40 min. Figure 4A shows the plasma clearance of Mn-PyC3A. Mn-PyC3A clears from plasma via a bi-exponential mechanism with comparable pharmacokinetics to Gd-DTPA. Mn-PyC3A and Gd-DTPA clear with distribution half-lives of 1.0±0.1 min and 1.1±0.7, respectively, and elimination half-lives of 31.7±4.4 and 22.8±13.3, respectively. Mn-PyC3A is metabolically stable. Mn-PyC3A was the only Mn containing species observed in the blood and urine. Figs 4B shows HPLC-ICP-MS Mn detection traces of plasma drawn at 1 min and 60 min following Mn-PyC3A injection, Fig 4C shows the urine Mn detection trace. A trace of a Mn-PyC3A standard is included in Fig4B-C for comparison.Conclusions
Mn-PyC3A is functionally equivalent to the GBCA Gd-DTPA in generating contrast for MR angiography at 3T. Mn-PyC3A is cleared via a mixed renal and hepatobiliary pathway. The mixed renal and hepatobiliary excretion of Mn-PyC3A indicate potential compatibility with renally impaired patients, as hepatobiliary clearance provides an elimination path when kidney function is low. Mn-PyC3A exhibited similar blood clearance to Gd-DTPA. Mn-PyC3A was excreted intact and no evidence of biotransformation was observed in the blood plasma or urine. The high stability of Mn-PyC3A, its similar relaxivity and pharmacokinetics to GBCAs, and its equivalent contrast enhanced MRA properties suggest that Mn-PyC3A is a promising candidate for clinical development as a non-gadolinium alternative to GBCAs.Acknowledgements
This work was supported by grants from the National Heart, Lung and Blood Institute (K25HL128899), The National Institute of Biomedical Imaging and Bioengineering (R21EB022804), the Boston Biomedical Innovation Center (U54HL119145), and instrumentation funded by the National Center for Research Resources and the Office of the Director (S10OD010650). We gratefully acknowledge Dr. Jacob Hooker for providing access to his baboon colony.References
1. Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21(4):1104-1108
2. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17(9):2359-2362.
3. Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015;276(1)228-232.
4. McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275(3):772-782.
5. Gale EM, Atanasova I, Blasi F, Ay I and Caravan P. A Manganese Alternative to Gadolinium for MRI Contrast. J Am Chem Soc 2015;137(49):15548-15557.
Figures