5566
Comparison of Gd-DTPA-BMA versus Gd-DOTA of Gadolinium retention in human bone tissue with renal function1Radiology, Kobe University Hospital, Kobe, Japan, 2Orthopedic Surgery, Kobe University Graduate School of Medicine, 3Radiology, Hyogo Prefectural Nishinomiya Hospital, 4Kobe Minimally Invasive Cancer Center, 5Kobe University Graduate School of Medicine
Synopsis
The purpose of this study was to determine the gadolinium (Gd) retention in human bone tissue after administration of Gd contrast agent such as macrocyclic (Gd-DOTA) or linear (Gd-DTPA-BMA) chelate at a standard single clinical dose and to evaluate its correlation with renal function.
Introduction
Recent reports suggest that nephrogenic systemic fibrosis (NSF) is associated with the administration of gadolinium (Gd) -based contrast agents in particular with the stability of Gd-complex1-3. It is reported that the stability of macrocyclic chelate is higher than linear chelate. Thus, high intensity on brain MR images is suggested to have a relationship with increased cumulative dose of linear Gd-based contrast agent in patients with normal renal function4,5. Previous study had reported on the Gd concentration remained in human bone tissue after administration of standard clinical doses6. However, this study did not determine the renal function in each patient. It is important to estimate Gd concentration compare to thier renal function.
Methods
Enrollment period: Jul 13, 2012 – Nov 03, 2016
Subjects: 14 patients (11 men and 2 women, aged between 10 to 47 years old) who underwent contrast MRI examination prior to surgical removal of a bone tumor, who met the following applicability. Patients were randomly divided into 2 groups for MR scan (Gd-DOTA administration group and Gd-DTPA-BMA administration group).
This study was approved by IRB in Kobe University.
Applicability criteria:
Patient who consent to this examination
Patient who underwent surgery 7-14 days after the MRI
Patient who was measured eGFR (hematology tests must be performed) 30 days before surgery
Patient who is a 10-65-year-old male or female with a good performance status
Patient who has no severe renal dysfunction (eGFR 30 mL/min/1.73 m3 or higher)
Six-month interval from the last Gd enhanced MRI
Exclusion criteria
Patient with contraindication criterias of contrast agents.
Too small samples to analyse.
Patient who did not consent to this study.
Patient who is unsuitable for this study decided by investigator
Contrast agents
GBCA (Gadlinium Based Contrast Agent)
Gadoterate (Gd-DOTA, Magnescope/Dotarem)
Gadodiamide (Gd-DTPA-BMA, Omniscan)
Dose 0.1 mmol Gd/kg (clinical dose) intravenous injection
Bone sampling
Bone tumor removed surgically within 7‑14 days after the contrast enhanced MRI.
Normal bone tissue in margin of tumor
Bone sample was restored at -20℃ until ICP-MS Gd measurement
Gd determination in bone
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Elan DRC+(PERKINELMER, INC)
Agilent 7700x(Agilent Technologies Inc.)
Internal standard (In, Rh, Tb)
Data analysis
Gd concentration in bone tissue
eGFR
The number of days from MRI scan to surgery
Age
Statistical analysis by unpaired t test (Gd-DOTA group vs Gd-DTPA-BMA group)
Results
Gd concentration and patients background are shown in Figure 1.
The mean values of Gd concentration in bone tissue, eGFR, interval MRI to surgery after single dose of Gd-contrast agent are shown in Figure 2.
Gd concentration in bone in Gd-DTPA-BMA group was significantly higher than that in Gd-DOTA group at single dose injection. No difference in renal function was found in both group.
Patients in the Gd-DTPA-BMA group showed 5.0 times higher concentration of Gd accumulated in human being bone that the Gd-DOTA group in patients with normal renal function.
Discussion
Gd chelate stability was reported in various Gd based contrast agents, due to chelate structure and ionicity. The stability constant of Gd-DTPA-DMA and Gd-DOTA are shown in Figure 3.
•The difference in clinical symptom (clinically significant effect of Gd deposition in bone tissue) was not found. This result is similar as reported in previous studies on brain MR image1,2).
•Linear Gd chelate showed higher accumulation of Gd than macrocyclic Gd chelate in normal renal function. The usage of Gd chelate should be carefully decided taking in consideration the age, disease, history of Gd enhanced scan (number of injection / type of Gd chelate / injection intervals / dose), and others.
•It remains unclear whether frequent Gd administration in patients with normal renal function is really tolerable, and whether the accumulation of Gd will consequently cause renal failure thus generating NSF.
Conclusion
In patients with normal renal function, typical linear Gd chelate, Gd-DTPA-BMA showed approximately 5.0 times more Gd accumulation in the human bone than macrocyclic Gd chelate, Gd-DOTA at a single dose application.Acknowledgements
No acknowledgement found.References
1. Abraham JL, Thakral C, Skov L, et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2007;158(2):273-280.
2. Port M, Idee JM, Medina C, et al. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008:21(4):469-490.
3. Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008 ;18(10):2164-2173.
4. Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material.Radiology. 2014;270(3):834-841.
5. Errante Y, Cirimele V, Mallio CA, et al. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49(10):685-690.
6. White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41(3):272-278.
Figures
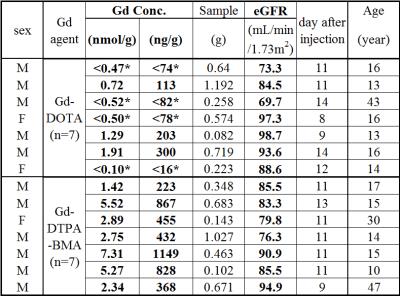
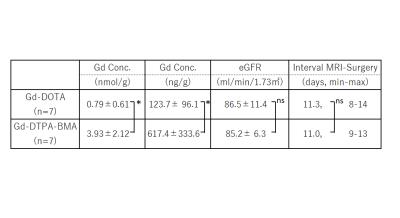
Table 2. Mean±SD, *: p<0.05 by t test
Note: the mean values calculated using upper values with LOQ.
