5563
In vivo measurements of gadolinium accumulation in bone of healthy individuals following administration of gadolinium-based contrast agents: a pilot study1Radiation Sciences Graduate Program, McMaster University, Hamilton, ON, Canada, 2Department of Physics and Astronomy, McMaster University, Hamilton, ON, Canada, 3Department of Physics, Ryerson University, Toronto, ON, Canada, 4McMaster School of Biomedical Engineering, McMaster University, Hamilton, ON, Canada, 5Department of Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada
Synopsis
The use of gadolinium (Gd) based contrast agents is being questioned due to its recently discovered retention in healthy individuals following administration. Our newest generation x-ray fluorescence system has been used in a small pilot study for in vivo Gd measurements in bones of healthy individuals, who have previously received these contrast agents. Preliminary results show a significant difference between the Gd-exposed and control groups, suggesting Gd accumulation in healthy individuals. Our system has performed the first human in vivo measurement of Gd in bone and has the potential to be used in further studies of accumulation in the body.
Purpose
The use of gadolinium (Gd) for enhancing contrast of MRI images has unquestionably been successful and commonly used world-wide over the past three decades. When first introduced, Gd-based contrast agents (GBCAs) were thought to be completely stable and non-metabolized (thus non-toxic), due to a chelating molecule. Furthermore, their high water solubility results in urinary excretion from the body within a few hours following administration 1,2. However, in 2006, GBCAs were linked to nephrogenic systemic fibrosis (NSF), suggesting the GBCA complex was breaking down and accumulating in the body 3-5. Recently, multiple studies have shown evidence of Gd accumulation in individuals with healthy renal function, raising concerns about the safety of GBCAs 6-10.
Bone serves as a storage site for Gd, as unusually high concentrations have been measured in individuals who had previously received GBCAs 11,12. All previous measurements of Gd in bone have been carried out using in vitro methods, which are invasive and can be painful. While there has been early evidence of intracellular Gd uptake 13, there has still been no in vivo study of Gd accumulation in bone in humans. We have developed a novel system to measure Gd in bone in vivo, to investigate the clinical implications of Gd accumulation in the body following administration of GBCAs.
Methods
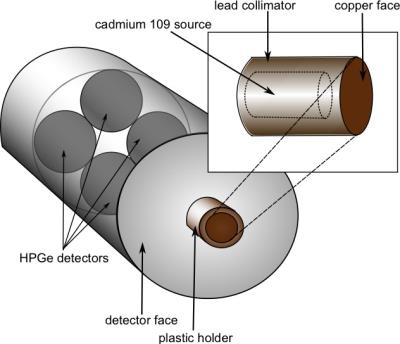
A small pilot study was conducted to test the ability of our self-designed x-ray fluorescence- based biomedical device (Figures 1 & 2) to detect Gd non-invasively and painlessly in the tibia of healthy volunteers. This study constitutes the first in vivo human measurement of Gd in bone. The population consisted of 10 healthy volunteers who had been administered various brands and amounts of GBCAs within the past 6 years, and 10 control subjects who have never received any contrast. Each volunteer was asked to sit for a measurement of their tibia using our x-ray fluorescence system. X-ray spectra were analyzed by fitting the Gd peaks with an algorithm to calculate peak area, then determining the Gd concentration from peak areas using a standard curve of calibration standards. Gd content from x-ray spectra were compared between the Gd-exposed and control groups using a t-test.Results
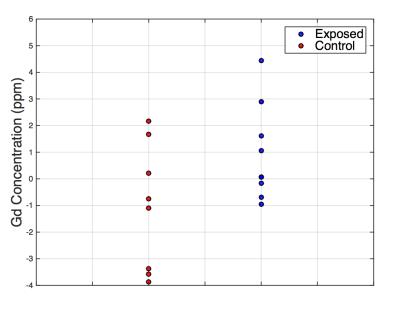
Preliminary results show a significantly elevated tibial Gd concentration (p<0.05) measured in the Gd-exposed group, relative to controls (Figure 3). In addition, we found two individuals within the Gd- exposed group who showed particularly higher Gd concentrations (by 2 and 3 standard deviations) compared to all others.Discussion
Our detection system can detect group differences in Gd concentration. The significantly higher Gd concentration suggests Gd accumulation is occurring in healthy individuals who had all been injected with various brands and amounts of GBCA. The strong Gd signals found in the two individuals may serve as interesting future cases to further our understanding of Gd accumulation in bone as a result of administration of GBCAs.Conclusion
Our x-ray fluorescence system is able to measure Gd in bone non-invasively and shows a significant difference in Gd concentration between the exposed and control group. This innovative system has the potential to be used in future research to explore clinical outcomes of using GBCAs, and to understand how GBCA brand and dosage affects the amount of Gd accumulated.Acknowledgements
No acknowledgement found.References
1. H.-J. Weinmann, R. Brasch, W. Press, G. Wesbey, Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent, Classic Papers in Modern Diagnostic Radiology (2005) 416–424.
2. D. Carr, J. Brown, G. Bydder, R. Steiner, H. Weinmann, U. Speck, A. Hall, I. Young, Gadolinium-DTPA as a contrast agent in MRI: initial clinical experience in 20 patients, American Journal of Roentgenology 143 (2) (1984) 215–224.
3. T. Grobner, Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?, Nephrology Dialysis Transplantation 21 (4) (2006) 1104–1108.
4. P. Marckmann, L. Skov, K. Rossen, A. Dupont, M. B. Damholt, J. G. Heaf, H. S. Thomsen, Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging, Journal of the American Society of Nephrology 17 (9) (2006) 2359–2362.
5. H. Thomsen, S. Morcos, P. Dawson, Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)?, Clinical radiology 61 (11) (2006) 905–906.
6. R. J. McDonald, J. S. McDonald, D. F. Kallmes, M. E. Jentoft, D. L. Murray, K. R. Thielen, E. E. Williamson, L. J. Eckel, Intracranial gadolinium deposition after contrast-enhanced MR imaging, Radiology 275 (3) (2015) 772–782.
7. C. C. Quattrocchi, C. A. Mallio, Y. Errante, V. Cirimele, L. Carideo, A. Ax, B. B. Zobel, Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy, Investigative radiology 50 (7) (2015) 470–472.
8. Y. Errante, V. Cirimele, C. A. Mallio, V. Di Lazzaro, B. B. Zobel, C. C. Quattrocchi, Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with nor- mal renal function, suggesting dechelation, Investigative radiology 49 (10) (2014) 685–690.
9. T. Kanda, K. Ishii, H. Kawaguchi, K. Kitajima, D. Takenaka, High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1- weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material, Radiology 270 (3) (2013) 834–841.
10. T. Kanda, T. Fukusato, M. Matsuda, K. Toyoda, H. Oba, J. Kotoku, T. Haruyama, K. Kitajima, S. Furui, Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy, Radiology 276 (1) (2015) 228–232.
11. T. H. Darrah, J. J. Prutsman-Pfeiffer, R. J. Poreda, M. E. Campbell, P. V. Hauschka, R. E. Hannigan, Incorporation of excess gadolinium into human bone from medical contrast agents, Metallomics 1 (6) (2009) 479–488.
12. N. Murata, L. F. Gonzalez-Cuyar, K. Murata, C. Fligner, R. Dills, D. Hippe, K. R. Maravilla, Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: Preliminary results from 9 patients with normal renal function., Investigative radiology 51 (7) (2016) 447–453.
13. Noseworthy, M. D., Ackerley, C., Qi, X., & Wright, G. A. (2002). Correlating subcellular contrast agent location from dynamic contrast-enhanced magnetic resonance imaging (dMRI) and analytical electron microscopy. Academic radiology, 9(2), S514-S518.
Figures