5559
Feasibility study for implementing low-field MRI with SPIO nanoparticles for endovascular interventions – An alternative to X-ray guided techniques1Faculty of Science and Technology, University of Twente, Enschede, Netherlands, 2Vascular Surgery, Medisch Spectrum Twente, Enschede, Netherlands
Synopsis
Low-field magnetic resonance imaging (lf-MRI) using super-paramagnetic iron-oxide (SPIO) nanoparticles as contrast agent seems to be a promising radiation free alternative to guide endovascular interventions in patients with critical limb ischemia (CLI). We propose an innovative workflow on how to deploy lf-MRI during such an intervention and investigate in phantoms the achievable contrast and resolution levels. The results showed that this combination of lf-MRI with SPIO contrast has potential to guide endovascular interventions, but that high quality pre-operative imaging might be required in deployment of this technique.
Purpose
Nowadays the preferred treatment of patients with critical limb ischemia (CLI) is minimally invasive endovascular revascularization using fluoroscopic imaging techniques enhanced with iodine contrast. X-ray is harmful for both the patient and the operating team. In addition, the used iodine contrast agent is harmful in nephrogenic patients and in patients with iodinated contrast allergy.
Low field magnetic resonance imaging (lf-MRI) using an open bore system has the potential to overcome the current disadvantages of fluoroscopic guided treatment of CLI patients, because it 1) is radiation-free, 2) offers positive contrast without adverse effects, and 3) provides maximum accessibility to the patient. The use of SPIO contrast agents at low magnetic field strengths to achieve positive contrast in arteries was investigated in static and dynamic circumstances. The purpose of this study was to investigate the workflow of an endovascular intervention guided by lf-MRI and which image quality could be reached.
Methods
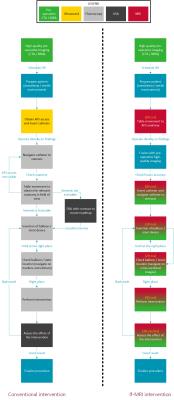
The proposed surgical workflow when using lf-MRI was adapted based on a state of the art hybrid operating room and currently used fluoroscopic workflow. The following steps were conducted in this research using the open configuration ESAOTE G-scan Brio 0.25 T MRI system:
A clinically relevant range1 of SPIO nanoparticles concentrations (0 mM – 4.096 mM) was prepared in small vials that were placed into chicken breast tissue to provide contrast with the background signal. The samples were scanned using a T1-weighted 2D spin echo (SE) sequence with a TR of 600 ms and a TE of 26 ms.
A flow phantom was made to mimic iliac and femoral mean blood flow (Figure 1). For background filling around the vessels a manganese-chloride (MnCl2) solution of 0.1 mM was used to correspond with soft tissue T1 and T2. An MnCl2 solution of 0.07 mM was used to correspond with blood T1 and T2. One fluid was prepared with only MnCl2, and to another one SPIO contrast nanoparticles (ferumoxytol, 60 μM Fe) were added. Both fluids were scanned using a 3D RF-spoiled gradient echo (SGRE) sequence with the following parameters: TR = 38 ms, TE = 16 ms, FA = 65°, acquisition matrix 192 x 128, FOV = 180 x 180 mm, reconstructed resolution = 0.7 x 0.7 x 0.7 mm. An additional 3D phase-encoding gradient was used in coronal direction with respect to the vessels.
A 3D reconstruction was made by subtraction of the scans without contrast from the scans with contrast. From these images a maximum intensity projection (MIP) was created using maximum slab thickness and a high strength sharpening filter with postprocessing software by TeraRecon (Aquarius v. 4.4, San Mateo, CA), that was visually assessed on usability and compared with conventional fluoroscopic images.
Results
We proposed an innovative workflow for performing an intervention on a patient with CLI using lf-MRI in comparison with the conventional fluoroscopy (Figure 2). In the static experiments with the SPIO range we found a positive contrast with a CNR higher than 5 in a range of 30 μM to 400 μM iron with a maximum CNR of 18 at 64 μM (Figure 3). Comparing coronal images from the series with and without SPIO contrast material showed a 236% increase in signal intensity (Figure 4). Digital subtraction of pre- and post-contrast series enhanced flow visualization (Figure 5). Visual assessment of 3D MIP images shows an adequate spatial resolution quality to visualize structures with sub-millimeter reconstructed resolution.Discussion
The levels of SPIO contrast agent required for positive contrast were found to be far below toxicity levels.1,2 The sub-millimeter reconstructed resolution of the subtraction and MIP images can be sufficient for guiding interventions, but these scans have acquisition times of more than one minute. This temporal resolution can be increased, but to preserve adequate spatial resolution, fusion with pre-operative high quality imaging is then recommended.3 Stenosis and structures of at least 1 mm must be distinguishable by the fused techniques to operate successfully and safely. Although we used pre-established concentration levels in phantom models, contrast injection techniques and contrast agent clearance rate determine the injection quantities in vivo.Conclusion
The proposed workflow can work in practice, with adequate spatial resolution using lf-MRI. However, temporal resolution should be increased to make this technique feasible for guiding endovascular interventions.Acknowledgements
No acknowledgement found.References
1 Walker J, et al. Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation of lower extremity arterial disease. Ann. Vasc. Surg. 2015;29(1):63-68.
2 Vasanawala S, et al. Safety and technique of ferumoxytol administration for MRI. Magn. Reson. Med. 2016;75(5):2107-2111.
3 Sailer A, et al. Fusion Guidance in Endovascular Peripheral Artery Interventions: A Feasibility Study. Cardiovasc. Intervent. Radiol. 2015;38(2):314–321.
Figures