5555
Clinical evaluation of automatic localization of prostate gold Fiducial Markers for MR-only Radiotherapy1Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, 2Cancer Center, UMC Utrecht, Utrecht, Netherlands
Synopsis
A novel approach aiming at automatic localization of gold Fiducial Markers (
Background
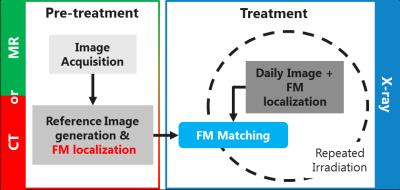
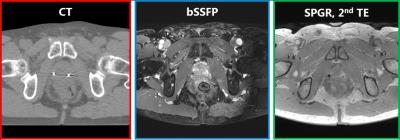
An MR-only Radiation Therapy Planning (RTP) workflow would reduce cost, radiation exposure and uncertainties introduced by CT-MRI registrations. In case of prostate RT (Figure 1), one of the main challenges is MR-based localization of intraprostatic gold Fiducial Markers (FMs), which is crucial for patient positioning. Currently, MR-based manual FM localization is clinically performed1. This, however, is sub-optimal as manual interaction potentially introduces variation and increases workload2. Attempts to perform automatic FM detection often rely on the signal void induced by the FMs in magnitude images which is non-specific3 (Figure 2), hampering accurate and robust automatic FM localization.Purpose
Here, we present an MR-only approach, based on a method previously proposed for brachytherapy seed localization4, that fully exploits the behaviour of the complex MR signal in the vicinity of susceptibility markers, aiming at localization of the gold FMs. Clinical evaluation of the proposed method was performed in terms of detectability, spatial accuracy and precision with respect to manual MRI-based and CT-based localization.Methods
Patient Data Collection
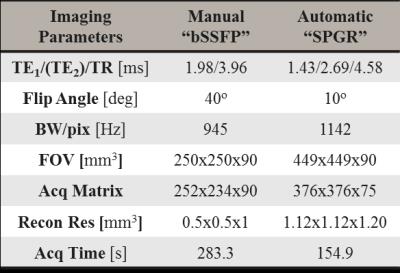
MR and CT (Figure 2) images of seventeen prostate cancer patients (61.5-82 yrs) who underwent standard RTP were collected. As part of routine clinical practice, three cylindrical gold FMs (length=5 mm, $$$\varnothing$$$=1 mm, HA2 Medizintechnik GmbH, Germany) were transperineally implanted one week prior to image acquisition. CT scanning (Brilliance CT, Philips, The Netherlands) was performed with a resolution of 1.1x1.1x3 mm3, followed by MRI within 1-2 hours (3T Ingenia, Philips, The Netherlands). Scan protocol included: 1) 3D balanced SSFP (bSSFP) sequence clinically used in RT planning to perform manual FM localization; 2) 3D RF spoiled GRE (SPGR) sequence for automatic FM localization.
CT-based FM localization was performed clinically in a semi-automatic fashion providing the Center of Gravity (CoG) and orientation for the FMs. To register MR to CT, MR-based manual FM localization (CoG only) was clinically conducted using the bSSFP data.
MR-based automatic Fiducial Marker localization
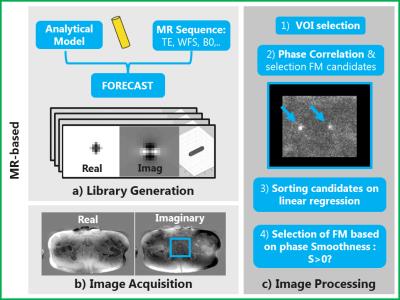
A 3D analytical model of the gold FM (χgold=-34 ppm) in a uniform background (χbck=-9 ppm) was used to simulate the field perturbation induced by a FM5. The complex signal behaviour in the proximity of a FM in the SPGR sequence was simulated at high resolution (0.07x0.07x0.08 mm3) and in a small FOV (23.6x23.7x25.2 mm3) using the FORECAST framework6 (Figure 3.1). A library of 321 templates with different FM orientations was generated. Finally, to locate FMs, a Phase Correlation (PC) matching7 between in-vivo and simulated template data was performed4, using a novel candidate selection criterion: FMs were selected only if the local Smoothness of the image phase $$$(S(\phi_I(x))=\sum_{I}\mid\frac{\partial e^{i\phi_I(x)}}{\partial x}\mid)$$$ along the readout direction $$$(x)$$$ was increased by template phase $$$(\phi_T(x))$$$ correction (Figure 3). For quality assurance purposes the algorithm was allowed to search for 4 FMs, even if only 3 FMs were implanted. The total time to simulate a library of templates was below 7 s, and the maximum time needed to localize FM on a single dataset was 20 s in Matlab (The MathWorks, USA) using a 16 GB RAM commercial CPU @3.4GHz.
Evaluation
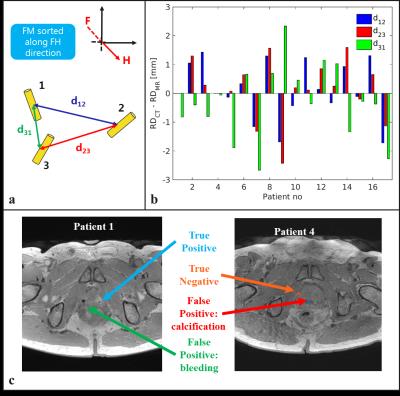
Manually and automatically detected FMs were labelled and coupled according to their CoG location in the craniocaudal direction (Figure 4a). Automatically detected FMs were regarded as correctly detected when the distance between coupled FMs was below 1.5 mm. To characterize the geometrical accuracy and precision of the MR-based localization, the Relative Distance (RD, see Figure 4a) of correctly detected corresponding FMs with the MR-based method (RD$$$_{MR}$$$) was compared to the RD on the gold standard CT (RD$$$_{CT}$$$).
Results
The proposed algorithm detected 3 FMs for all the patients, except for patient 1 where only 2 FMs were detected. Although allowed for in the method, a 4th FM was not detected in all the patients thanks to the smoothness criterion. The method correctly detected 49/51 FMs resulting in a detection rate of 0.96. The mean, median and STD of the RD$$$_{CT}-$$$RD$$$_{MR}$$$ over all the patients of the correctly detected FM was 0.0, 0.2, and 1.2 mm, respectively (Figure 4b).Discussion and Conclusion
The proposed method automatically localized the FMs with high accuracy, and precision. In one case a missed detection originated from a bleeding in the direct vicinity of the FM which generated a larger artefact, and, in another case, a false-positive detection originated from calcifications (Figure 4c). The method was found to be spatially accurate, which is essential for clinical use. To overcome any missed detection, we envision the use of the proposed method along with verification by an observer. This will result in a semi-automatic workflow facilitating the introduction an MR-only workflow.Acknowledgements
The research is funded by ZonMw IMDI Programme, project number: 10-104003010. The project is co-funded by Philips Healthcare.References
[1] De Los Santos J, Popple R, Agazaryan N, et al. Image Guided Radiation Therapy (IGRT) Technologies for Radiation Therapy Localization and Delivery. Int J Radiat Oncol. 2013;87(1):33-45.
[2] Ghose S, Mitra J, Rivest-Hénault D, et al. MRI-alone radiation therapy planning for prostate cancer: Automatic fiducial marker detection. Med Phys. 2016;43:2218-2228.
[3] Chan MF, Cohen GN, Deasy MSJO, Qualitative Evaluation of Fiducial Markers for Radiotherapy Imaging, Technol Cancer Res Treat. 201;14(3):298-304.
[4] Zijlstra F, Bouwman JG, Moerland MA, Seevinck PR. Fully automatic in-vivo localization of LDR brachytherapy seeds for post-implant dosimetry using MR simulations and template matching, Proc Intl Soc Mag Reson Med 24. 2016;3615.
[5] Bouwman JG, Bakker CJ. Alias subtraction more efficient than conventional zero-padding in the Fourier-based calculation of the susceptibility induced perturbation of the magnetic field in MR. Magn Reson Med. 2012;68(2):621-30.
[6] Zijlstra F, Bouwman JG, Braškute I, et al. Fast Fourier-based simulation of off-resonance artifacts in steady-state gradient echo MRI applied to metal object localization. Magn Reson Med. 2016;in press.
[7] Kuglin CD, Hines DC. The phase correlation image alignment method. IEEE Trans Cybern. 1975;163-165.
Figures