5551
An Approach for Accurate Quantification of Hepatic Metastatic Burden during MRI-Guided Laser Ablation: Impact on Management Decisions in 41 Patients1Radiology and Imaging Sciences, Emory University Hospital, Atlanta, GA, United States, 2Interventional MRI Program, Emory University Hospital, Atlanta, GA, United States, 3School of Medicine, Emory University, Atlanta, GA, United States
Synopsis
Treatment of patients with metastatic liver disease requires accurate quantification of hepatic tumor burden and precise three dimensional localization. We demonstrate that intraprocedural MRI utilizing IV gadoxetate disodium (Eovist®) administration with controlled breath suspension under general anesthesia results in the detection additional hepatic metastatic deposits in 25% of cases, not appreciated on prior diagnostic imaging. In 88% of these cases this discovery led to a change in clinical management strategy that may have influenced patient outcomes.
Introduction
The liver is among the most common sites for metastatic disease to manifest, accounting for 25% of distant solid organ metastasis.1 Treatment of patients with metastatic liver disease requires accurate quantification of tumor burden, proper multidisciplinary planning, and access to reliable minimally invasive modes of loco-regional tumor control.2-3 The current standard of care available falls short in two aspects of this process: a) providing consistent pre-operative detection of subtle disease, often requiring direct intra-operative ultrasound of the exposed liver prior to metastatectomy; and b) allowing unambiguous identification of subtle metastases for precise targeting when percutaneous ablation is planned. These limitations lead to hepatic resections and/or open ablations that maybe avoidable, particularly in poor surgical candidates. The aim of this study is to assess the value of a modified technique for MRI-guided laser ablation of liver metastases utilizing intra-procedural IV gadoxetate disodium (Eovist®) administration and controlled breath suspension under general anesthesia for a) detecting subtle metastases not seen on pre-procedure scans; b) facilitating precise targeting of small lesions; and c) enhancing the safety of ablation near central bile ducts.Materials and Methods
A retrospective analysis of patients that underwent MRI guided liver laser ablations was performed encompassing the period of January 2012 to December 2015. Patient baseline characteristics, primary diagnosis, pre-procedural diagnostic imaging results, intraprocedural imaging and procedural results were examined. Patients were referred for MRI guided intervention because of the subtle nature of metastases, challenging location, and/or the desire to assess for further disease not seen on pre-procedure diagnostic MRI scans. Procedures were performed within an interventional MRI suite equipped with 1.5T wide bore scanner. Interventions were performed under general anesthesia within the scanner bore while viewing real-time image updates on an in-room monitor. Initially, IV Eovist®(0.025 mmol/kg) was administered and whole liver imaging was performed using VIBE (TR/TE 4.19/2.1), TSE T1(TE/TE=436/4.4) and TSE T2 (TE/TE=3000/84) in 3 planes during suspended breathing. Once the metastatic burden has been confirmed/updated, an interactive visualization on a tri-orthogonal plane FLASH sequence (TE/TE=1220/1.92) was used to guide a laser fiber with 15mm diffusing tip encased in 5.5 F cooling catheter (Visualase, TX) into the target lesion(s). A test dose of diode laser energy (980nm,30sec,4.5W) was applied to verify the location of ablation nidus on real-time temperature and cumulative damage estimate mapping(TE/TE=24/10). Subsequently, ablative energy dose was delivered with treatment endpoint based on on-line thermal monitoring of growing ablation. Fiber repositioning for additional ablation was conducted as needed. Final ablations were evaluated on a repeat set of pre-ablation scans with VIBE and TSE T1 scans repeated after a final dose of MultiHance (0.1 mmol/kg).Results
41 patients (22M, 19F, average ages 60 and 61 years, respectively) ecompassing 63 independent liver ablations are included in this analysis. The cohort included metastatic colon adenocarcinoma (48.8%, n=20), metastatic pancreatic adenocarcinoma/acinar cell/neuroendocrine (26.8%, n=11), metastatic rectal adenocarcinoma (7%, n=3), metastatic renal cell (2.4%, n=1), hepatocellular carcinoma (2.4%, n=1), metastatic breast adenocarincoma (2.4%, n=1), clear cell carcinoma of the stomach (2.4%, n=1), metastatic cholangiocarcinoma (2.4%, n=1), metastatic hemangiopericytoma (2.4%, n=1) and metastatic GIST tumor (2.4%, n=1). Intra-procedural imaging with the hepatocyte-specific contrast agent under controlled suspended breathing allowed accurate mapping of metastatic tumor burden within the liver prior to proceeding with the planned ablations. In 22.2% of cases (n=14 of 63), this technique resulted in identification of new hepatic metastatic foci that led to changes in management, including additional rounds of same session targeted ablation (57%, n=8), additional sessions of ablation scheduled at a later date (14%, n=2), abortion of procedure (14%, n=2), and close short term follow-up with additional previously unscheduled imaging (14%, n=2). In 3% of total ablations (n=2), additional lesions were found; however, ablation attempts were not made due to excessive tumor burdens.Discussion
This investigation reports a comprehensive approach for minimally invasive MR-based loco-regional control of hepatic metastases, with particular value in cases of occult liver lesions. The application of hepatocyte-specific contrast during high resolution MR imaging under GA controlled suspended breathing prior to focal ablation is analogous to using intra-operative ultrasound for definitive mapping of metastatic burden prior to hepatic metastatectomy. Utilizing this approach in our series, 25% of the lesions were subtle additional metastases not identified on prior imaging. In 88% of these cases, the discovery of additional nodules resulted in a change in management which was defined as additional rounds of same session targeted ablation, additional ablations scheduled at a later date or abortion of the procedure.Acknowledgements
No acknowledgement found.References
1. Abbruzzese JL, Abbruzzese MC, Lenzi R, et al. Analysis of a diag- nostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094-2103.
2. Kalra N, Gupta P, Chawla Y, et al. Locoreginal treatment for hepatocellular carcinoma: The best is yet to come. World J Radiol, 2015;7(10);306-318
3.Tatli S, Morrison PR, Tuncali K, et al. Interventional MRI for oncologic applications. Tech Vasc Interv Radiol, 10.2 (2007): 159-170
Figures
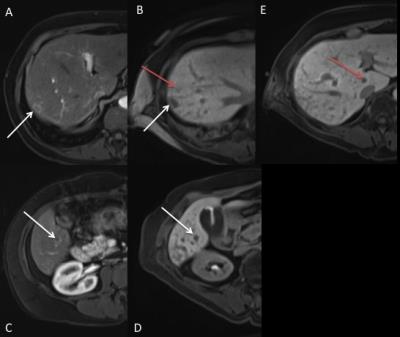
Figure 1: 59-year-old female with metastatic colorectal cancer. Comparison of corresponding regions on previous diagnostic imaging (post-contrast VIBE sequences, A&C) versus preprocedure MRI (VIBE with Eovist sequences, B,D&E)
Images A&B demonstrate an arterially enhancing lesion in segment 7 seen in both settings (White arrow). Image B demonstrates a lesion not seen on prior diagnostic imaging (Red Arrow). Images C and D demonstrate an arterially enhancing lesion in segment 5, seen both settings. Image E demonstrates a hypointense lesion in the caudate lobe (Red Arrow), likely representing a metastatic deposit in the caudate lobe, also not seen on prior diagnostic imaging.
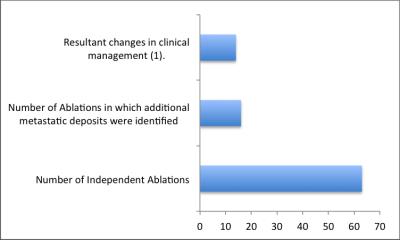
Figure 2: Breakdown of independent ablation events that led to the discovery of additional hepatic metastatic deposits not appreciated on previous diagnostic imaging and proportion of those events the led to a change in clinical management.
1: Defined as additional rounds of same session targeted ablation, additional sessions of ablation scheduled at a later date, abortion of procedure and close short term follow-up with additional previously unscheduled imaging.