5550
Improved planning of MR-HIFU therapy for breast cancer using image registration of pre- and per- treatment mDixon MRI1UMC Utrecht, Utrecht, Netherlands
Synopsis
During Magnetic Resonance Imaging-guided High Intensity Focused Ultrasound (MR-HIFU) ablation of breast tumors, localization of the tumor during the treatment procedure is important for proper treatment planning. However, the use of a contrast agent during thermal ablation is preferably avoided for reasons of safety and practicality. We propose an image registration approach using pre-treatment eligibility CE and per-treatment non-CE breast MR scans, acquired with a dedicated mDixon-based tumor localization scan. We demonstrate the feasibility of our method in a volunteer study.
Introduction
Magnetic Resonance Imaging-guided High Intensity Focused Ultrasound (MR-HIFU) has been proposed for minimally invasive thermal ablation of breast tumors.1,2 Typically, the treatment planning stage of a breast MR-HIFU procedure has 2 phases: first, a fat-suppressed contrast-enhanced (CE) scan is made to assess patient eligibility.3 To ensure adequate image quality and tumor visualization, this scan is made with a dedicated breast coil on a 3-T scanner. The second phase is tumor localization during the treatment procedure, with the patient in treatment position on the MR-HIFU system. Then, localization of the tumor is preferably done without a contrast agent, because of concerns related to the trapping of the contrast agent due to the thermal ablation4 and the inability to repeat contrast-enhanced scans, e.g. in case of patient motion, due to dose limitations.
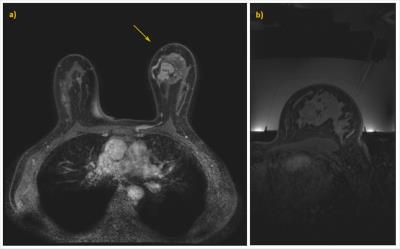
Image registration could provide a solution. Registration of the fat-suppressed eligibility CE-MRI scan with non-CE per-treatment images is challenging because of differences in contrasts between the scans, particularly inside the tumor and because of water surrounding the breast for acoustic coupling during therapy. On the other hand, without fat-suppression, the ability to separate tumor from fat would be compromised. Additionally, the breast deformation between the eligibility scan, with free hanging breasts, and the per-treatment scan with the breast in a water-filled cup can be severe (figure 1).
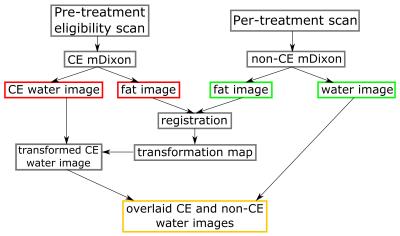
We propose a solution for this planning problem, using image registration on CE and non-CE scans acquired during both phases of the treatment planning respectively, with a dedicated mDixon-based tumor localization scan.
In this approach simultaneously acquired water and fat data complement each other. Registration is done on fat images, which will not be influenced by the contrast agent in the eligibility scan, nor by the water in the cup outside the breast in treatment position. After registration, an overlay of the corresponding water image allows visualization of the CE tumor onto the non-CE per-treatment scan. We demonstrate the feasibility of the image acquisition and registration approach in a volunteer study.
Methods
The proposed workflow is shown in figure 2.
All experiments were performed on a clinical 3-T MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands). Six healthy female volunteers were scanned. Sagittal 3D T1-weighted FFE mDixon scans were performed: TR=6.3 ms, TE=3.3 ms, dTE=0.8 ms, FA=12°, WFS=0.605 pixels, FOV=280x340x150 mm3, voxel size=1.2x1.2x1.2 mm3.
In each volunteer, one breast was imaged. Scans were repeated 3 times, with different deformations: I) a scan wearing a t-shirt (modeling breast in treatment position inside a HIFU breast cup filled with water (see figure 1b)), II) a scan with a free hanging breast in a dedicated breast coil (as during the eligibility scan (see figure 1a)) and III) a scan with more severe deformations, created by adding sandbags to the breast coil.
Fat images of the eligibility scans and the per-treatment scans were registered using a normalized cross-correlation metric (elastix5,6) in two steps: an affine registration followed by a b-spline registration.
Since there were no tumors in the volunteer scans, the tumor target registration error could not be evaluated. Therefore, evaluation was done by calculating the DICE similarity index for registered fat and water images separately, using an Otsu threshold7 in an ROI containing only the breast.
Results
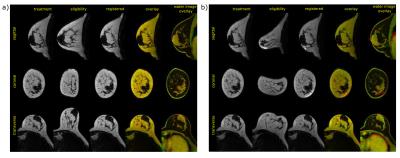
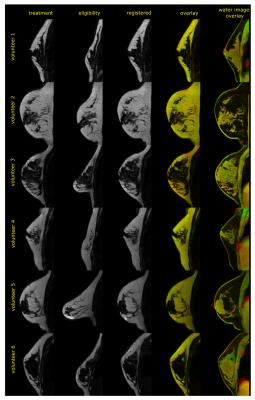
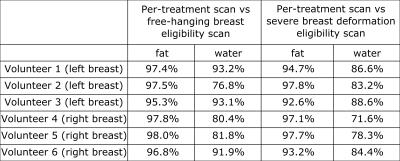
Excellent alignment between eligibility scans (free-hanging breast) and per-treatment scans (volunteer wearing a t-shirt) was observed (figure 3a). Even with severe breast deformation reasonable results were obtained (figure 3b), for all volunteers (figure 4).
This is confirmed by high DICE similarity index values (figure 5).
Discussion
We have demonstrated that fat images taken from an mDixon series can be used for breast registration between eligibility scans and per-treatment scans. There is evidence that CE mDixon can be used for tumor depiction in the breast.8,9 When applied in patients, the eligibility scan would show contrast enhancement of the tumor in the water image only, leaving the fat image useful for registration.
As expected, registration was better in the more realistic free-hanging breast case, than with extreme deformations.
In practice, because of the inferior receiver coil and lower field strength of the MR-HIFU system, treatment scans will be of lower quality that those obtained at a 3-T scanner with a dedicated breast coil. Therefore, having registered data from a higher-quality 3-T scan available during the treatment is an advantage.
In patients the most relevant measure of performance will be the target registration error. For that, further investigations in patients with contrast enhancement of the tumor is needed.
The proposed method may also be valuable for other image-guided treatments, such as MR-guided radiotherapy.
Acknowledgements
No acknowledgement found.References
1. van den Bosch, M., Daniel, B., Rieke, V. et al. J. Magn. Reson. Imaging (2008) 27: 204–208.
2. Merckel, L.G., Bartels, L.W., Köhler, M.O. et al. Cardiovasc Intervent Radiol (2013) 36: 292.
3. Merckel, L.G., Knuttel, F.M., Deckers, R. et al. Eur Radiol (2016) 26: 4037.
4. Hijnen, N. M., Elevelt, A. and Grull, H. Invest Radiol (2013) 7: 517-524.
5. Klein, S., Staring, M., Murphy, K. et al. IEEE transactions on medical imaging (2010) 29(1): 196-205.
6. Shamonin, D. P. et al. Frontiers in Neuroinformatics (2014) 7(50): 1-15.
7. Otsu, N. IEEE Trans. Sys., Man., Cyber (1979) 9(1): 62–66.
8. Le-Petross, H., Kundra, V., Szklaruk, J. et al. J. Magn. Reson. Imaging (2010) 31: 889–894.
9. Saranathan, M., Rettmann, D. W., Hargreaves, B. A. et al. J. Magn. Reson. Imaging (2014) 40: 1392–1399.
Figures