5546
Advanced Passive Tracking and Visualization of MR-Compatible Diagnostic Electrophysiology Catheter1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Imricor Medical Systems, Burnsville, MN, United States, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
MRI shows promise for the guidance of electrophysiology (EP) procedures. MR-guided EP procedures require reliable catheter tracking capabilities. Passive catheter tracking enable positive or negative contrast visualization of the catheter in the MR-images using for example integrated ferromagnetic/paramagnetic materials or contrast agent. Positive contrast visualization remains challenging and often sensitive to imaging/post-processing parameters. Negative contrast techniques remain associated with confounding factors (i.e. any other signal void) which complicate visual catheter tracking. In this study, we sought to develop and evaluate a novel framework for passive catheter tracking with negative contrast combined with automatic tracking and enhanced visualization of the catheter.
Background
MRI shows promise for the guidance of electrophysiology (EP) procedures1. MRI-guided EP procedures require reliable catheter tracking capabilities. Active catheter tracking can be used if the diagnostic EP catheter has integrated microcoils2. However, such additional hardware substantially increase catheter cost and catheter diameter (which may complicate catheter access when using multiple catheters). Passive catheter tracking is an alternative approach which enables positive or negative contrast visualization of the catheter in the MR-images using for example integrated ferromagnetic/paramagnetic materials3 or contrast agent4. Positive contrast visualization remains challenging and often sensitive to imaging/post-processing parameters. Negative contrast techniques remain associated with confounding factors (i.e. any signal void in the images) which complicate visual catheter tracking. In this study, we sought to develop and evaluate a novel framework for passive catheter tracking with negative contrast combined with automatic tracking and enhanced visualization of the catheter. This approach was evaluated during an MR-guided EP procedure in swine.Methods
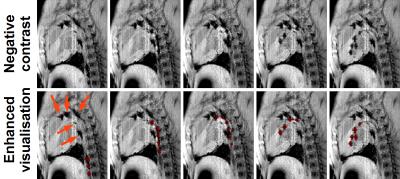
Proposed passive catheter tracking framework. Passive catheter tracking with negative contrast was achieved using a spoiled gradient echo imaging sequence with a water-selective excitation pulse (binomial first order). In addition to the suppression of the fat signal, the intrinsic longer echo time of this excitation pulse served to increase the signal dephasing and resulting signal void artefacts near the catheter markers. Automatic tracking of the catheter markers was achieved using a prototype algorithm by combining anatomical images acquired before the insertion of the catheter and negative contrast images of the catheter as follows. The difference between anatomical and negative contrast images is first computed. Catheter markers are then automatically identified by binary segmentation of the difference image (threshold of 10% of maximum signal intensity). Erosion and dilatation operators are then performed to remove isolated outliers. The remaining signal is finally overlaid to the negative contrast images to facilitate catheter marker identification and catheter navigation.
Experimental validation: The proposed passive catheter tracking framework was evaluated during MR-guided EP procedure in one swine. The animal was anesthetized and mechanically ventilated. Imaging was performed using a 1.5T Magnetom Aera scanner (Siemens Healthcare, Erlangen, Germany). The passive catheter tracking sequence used real-time single-shot spoiled gradient echo imaging with the following parameters: TR/TE=8.3ms/4ms, flip angle=15°, FOV=340×250mm2, voxel size=1.6×1.6mm2, slice thickness=10mm, slice number=4, bandwidth=250Hz/Px, GRAPPA factor=2, binomial water excitation pulse). The diagnostic EP catheter was a 7 French MR-compatible catheter with integrated “passive markers” (Vision-MR Diagnostic Catheter, Imricor, Burnsville, MN). The quality of negative contrast images was first evaluated in-vivo during MR-guided catheter navigation. The full framework was evaluated during post-mortem catheter navigation to show its initial feasibility in the absence of physiological motion.
Results
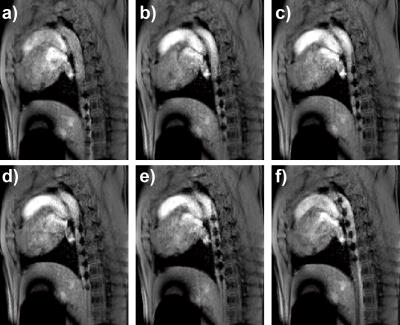
Figure 1 shows in-vivo negative contrast images acquired in one swine. This sequence provided excellent simultaneous visualization of anatomy and catheter. The catheter markers and tip could be well visualised and appeared with high contrast in all the images. Figure 2 shows the proposed passive tracking framework where the catheter can be visualised with negative contrast. The catheter markers were successfully identified and segmented using the proposed approach and are overlaid in red for improved visualisation. These markers were correctly identified and segmented in most images. Signal void artefacts unrelated to the catheter (orange arrows) were correctly discarded from the segmentation of the catheter markers.Discussion
The proposed framework and its enhanced visualization may help removing any signal ambiguity between catheter and other signal void artefacts. This framework was successfully validated post-mortem. Its in-vivo translation will requires integration of a motion correction step to account for physiological motion between anatomical and negative contrast images. Further studies are warranted to evaluate the in-vivo benefit of the proposed framework to improve MR-guided catheter navigation.Conclusions
Excellent in-vivo catheter visualisation was achieved using the proposed negative contrast sequence. The proposed framework enabled successful post-mortem real-time catheter navigation with automatic tracking of catheter markers and enhanced catheter visualization.Acknowledgements
This work was supported by the Health Innovation Challenge Fund [Grant number HICF-R10-698], a parallel funding partnership between the Department of Health and the Wellcome Trust. This research was also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London, and by the NIHR Healthcare Technology Co-operative for Cardiovascular Disease at Guy’s and St Thomas’ NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] Nazarian S. et al., Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation 2008; 118:223-9
[2] Dumonlin CL, et al., Real-time position monitoring of invasive devices using magnetic resonance. Magn Reson Med. 1993 Mar;29(3):411-5
[3] Rubin DL, et al., Magnetic susceptibility effects and their application in the development of new ferromagnetic catheters for magnetic resonance imaging. Invest Radiol. 1990 Dec;25(12):1325-32.
[4] Omary RA, et al., Real-time MR imaging-guided passive catheter tracking with use of gadolinium-filled catheters. J Vasc Interv Radiol. 2000 Sep;11(8):1079-85.
Figures