5542
Unseen Clinically Significant Prostate Cancer on 3T Multiparametric MRI Challenging Screening and Focal Therapy: An In-bore MRI-Guided Biopsy Study of MRI Negative Areas1Radiology, Emory University-School of Medicine, Atlanta, GA, United States, 2Urology, Emory University-School of Medicine, Atlanta, GA, United States, 3Hematology-Oncology and Urology, Emory University-School of Medicine, Atlanta, GA, United States, 4Radiation Oncology, Emory University-School of Medicine, Atlanta, GA, United States
Synopsis
The use of multiparametric MRI for prostate cancer screening is challenged by the potential missing of clinically significant cancer in areas with no visible abnormalities. We randomly biopsied areas with no visible targets under direct MRI guidance. Out of 43 biopsied areas, negative predictive value for clinically significant cancers was 90.7% suggesting a very low potential for harboring clinically significant cancers and supporting the use of mpMRI in cancer screening and active surveillance. An extended biopsy approach including sampling of areas without visible MRI abnormalities may still need to be considered prior to focal therapy.
Introduction and Purpose:
There has been a progressive rise in the utilization of multiparametric MRI (mpMRI) in prostate cancer screening. The ability to visualize suspicious targets paved the road to MRI targeted biopsies and targeted focal therapies. Although mpMRI shows high negative predictive value for clinically significant cancers, MRI areas with no definite abnormalities might harbor clinically significant prostate cancers1-4. Therefore, restricting biopsies to MRI visible targets only may lead to inappropriately assigning patients to active surveillance or erroneously selecting them for focal therapy. The purpose of this study is to investigate the outcomes of added random biopsies from MRI negative areas in the setting of MRI guided biopsies obtained from other MRI visible targets.Methods:
We retrospectively analyzed 178 cases who had multiparametric MRI and subsequent in bore Transrectal MRI guided biopsy (MRGB) at our institution from 2013 through 2016. We selected those who had biopsies for MRI negative areas (defined as those having PI-RADS V2 category 1 or 2 with no definite focal target).5 The final population included in the analysis was 27 patients with 43 sampled MRI negative areas. All cases had mpMRI in a separate session prior to the MRGB using 3T MRI scanner (Magnetom Trio, Siemens, Germany) with 32 channel surface phased array pelvic coil in supine position to obtain high resolution axial, sagittal, and coronal TSE T2-WIs, Axial DWI (b-values 1500 - 2000 s/mm2), DCE-MRI and pre- and delayed post-gadolinium VIBE scans. Transrectal in bore MRI guided biopies were performed in the prone position on the same MRI scanner using the Dyna-TRIM/DynaCAD system (Invivo, Gainesville, FL ). During the biopsy sessions, target selection was guided by DWI and T2WI and cross matched with images from the prior diagnostic mpMRI. Each sampled area was reported as “having no image correlate” and labeled the same identifying number across both the MRGB and pathology reports. Areas were selected on random basis. Clinically significant cancers were defined as those with Gleason score ≥7. The pathology results were referenced to MRGB. Results were evaluated for 1) spectrum of pathological outcomes; 2) presence of cancer, its grade and volume; and 3) negative predictive value (NPV) for all prostate cancers as well as for clinically significant cancers. Descriptive statistical analysis was done using SPSS (version 23, IBM) for Microsoft Windows. Categorical variables were reported in frequency and percentages, quantitative variables were summarized in medians, IQRs and ranges.Results
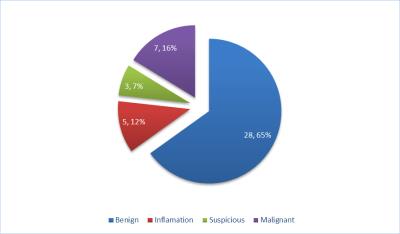
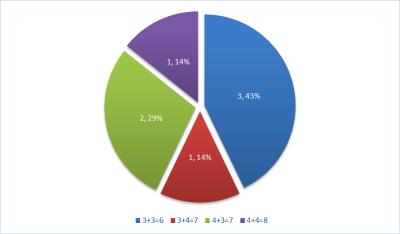
Twenty-Seven patients met our inclusion criteria for this study with median age of 65 years (IQR 61-69) and median PSA of 6.7 ng/mL (IQR:5-10). 18 patients had prior TRUS biopsies (TRUS-Bx). Number of MRI visible targets biopsied per patient ranged between 1-4 targets and number of MRI negative areas biopsied ranged between 1-2 areas per patient. 22 patients (81.5%) were diagnosed with prostate cancer either with MRI visible targets alone (13/22), TRUS-Bx Alone (4/22), both MRI visible targets and TRUS-Bx (4/22) and MR guided biopsy of MRI negative areas only (1/22). MRI negative areas showed higher grade than MRI targets in one patient (MRI Targets =4+3=7 vs MRI negative areas =4+4=8) (4.5%) and diagnosed cancer not detected through biopsied MRI targets in one patient (3+3=6). Out of 43 biopsied MRI negative areas, there were 7/43 (16%) malignant lesions (false negative mpMRI). NPV of mpMRI for all cancers was 84%, and for clinically significant cancer was 90.7%. Different pathological outcomes for MRI negative areas are illustrated in figure 1. Most of MRI negative areas with malignant pathology results had clinically significant cancers (4/7, 57%) (Figure 2). In cases that had MRI visible targets, most of MRI negative areas having cancers were contra-lateral to those MRI targets (3/5, 60%). The median cancer core % length was 40 % (range 5-90%).Discussion and Conclusion:
MRI negative areas (PI-RADS V2 category 1 or 2 with no definite focal target) demonstrated a high negative predictive value for clinically significant cancers (90.7%) in keeping with previous reports. 1, 2 These results support the use of mpMRI in prostate cancer screening and active surveillance. An extended biopsy approach including sampling of areas without visible MRI abnormalities may still need to be considered prior to focal therapy.Acknowledgements
No acknowledgement found.References
1. Wysock JS, Mendhiratta N, Zattoni F, Meng X, Bjurlin M, Huang WC, et al. Predictive Value of Negative 3T Multiparametric Prostate MRI on 12 Core Biopsy Results. BJU Int. 2016.
2. De Visschere PJ, Naesens L, Libbrecht L, Van Praet C, Lumen N, Fonteyne V, et al. What kind of prostate cancers do we miss on multiparametric magnetic resonance imaging? Eur Radiol. 2016;26(4):1098-107.
3. Tan N, Margolis DJ, Lu DY, King KG, Huang J, Reiter RE, et al. Characteristics of Detected and Missed Prostate Cancer Foci on 3-T Multiparametric MRI Using an Endorectal Coil Correlated With Whole-Mount Thin-Section Histopathology. AJR Am J Roentgenol. 2015;205(1):W87-92.
4. Rastinehad AR, Waingankar N, Turkbey B, Yaskiv O, Sonstegard AM, Fakhoury M, et al. Comparison of Multiparametric MRI Scoring Systems and the Impact on Cancer Detection in Patients Undergoing MR US Fusion Guided Prostate Biopsies. PLoS One. 2015;10(11):e0143404.
5. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16-40.