5541
Hemorrhage Events during MR Guided DBS Implantations1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Neurological Surgery, UCSF, 3Neurology, UCSF
Synopsis
MR guidance is increasingly being used to implant DBS electrodes. The technique is extremely accurate and permits patients to be under general anesthesia during the procedure. The incidence of complications during these procedures, however, has not been established. We report on the incidence of hemorrhagic events during 231 surgical procedures (374 electrodes implanted). The ability to detect hemorrhage intra-operatively is demonstrated and factors contributing to hemorrhage incidence are identified. Total hemorrhage rates and symptomatic hemorrhage rates were found to be 2.4%/electrode implanted and 1.1%/electrode implanted respectively, which is comparable to conventional surgical approaches for DBS implantation.
PURPOSE
This study reports on the incidence of hemorrhage during MR guided DBS implantations. The ability of intra-operative MR imaging to detect and characterize hemorrhage is evaluated. Factors contributing to hemorrhage risk are identified and the achieved rates are compared against conventional surgical approaches to DBS implantation.METHODS
MR guided DBS implantations were performed in 231 surgical procedures (374 implanted electrodes). All procedures were performed under IRB approved protocols on patients with movement disorders including Parkinson’s disease (PD), dystonia, Tourette’s syndrome, or nonparkinsonian tremor disorders. Stimulation targets were predominantly the subthalamic nucleus (STN) or the globus pallidus interna (GPi). Implantations were performed with a skull mounted trajectory guide (Medtronic NexFrame or MRI Interventions SmartFrame), following principles outlined elsewhere [1,2]. Importantly, the brain was penetrated with a rigid ceramic or titanium mandrel (1.4mm in diameter, 305mm in length) within a peel-away sheath. After confirming acceptable placement, the rigid mandrel was then withdrawn from the peel-away sheath and replaced with a DBS electrode (Medtronic DBS Model 3389-28 or 3389-40). Finally, the peel-away sheath was removed, leaving just the DBS electrode, which was anchored to the skull. Hemorrhage was detected with intraoperative or post-operative MR imaging or post-operative CT and was classified based on its point of origin and clinical impact.RESULTS
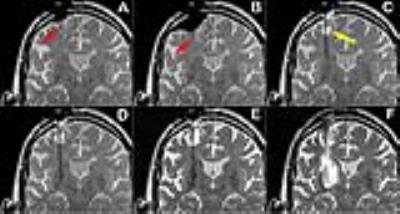
The NexFrame system was used in 49 surgeries (82 electrodes), and the SmartFrame was used in 182 surgeries (292 electrodes). A ceramic mandrel was employed for all SmartFrame procedures. Multiple insertions into the brain were required in 16% of electrodes implanted with the NexFrame system, and 2% of the electrodes implanted with SmartFrame. Hemorrhage was detected in nine patients, providing an overall intracranial hemorrhage rate of 2.4%/electrode or 3.9%/surgery. Hemorrhage was subdural/subarachnoid in three cases, subcortical in five cases, and deep in one case. Hemorrhage was symptomatic and clinically significant in four cases (1.1%/electrode, 1.7%/surgery). The average age of patients with hemorrhage (64.8±6.3) was older than the average patient age (57.9±16.7), which was statistically significant (P=0.0125). Blood pressure is another known risk factor and was below 140mm Hg at the time of all insertions. Hemorrhage was readily detected with T2-weighted intra-operative MR imaging and seven of the hemorrhages were detected in this fashion. Hemorrhage origin was subdural/subarachnoid (2) subcortical in (4) or deep (1) in these cases and two proved to be clinically significant. Factors believed to have contributed to hemorrhage included crossing a sulcus and resistance at the pial membrane. The latter produced a rebound hemorrhage (Figure) and led to a change in clinical practice. A sharp insertion mandrel is now initially used to cross the cortical surface, before exchanging for a blunt mandrel for the remainder of the insertion through the brain. Similar rebound hemorrhages have not occurred since this change in practice. Two hemorrhages occurred in the post-operative period and were associated with acute neurological decline and hemorrhage was evident on post-operative CT.DISCUSSION
Hemorrhage incidence in conventional DBS surgery has been reported to be 5.0%/surgery, with 1.9%/surgery being asymptomatic [3]. Our rates of 3.9%/surgery, with 1.7%/surgery being symptomatic, are comparable to literature values. Operating within a magnet bore poses some challenges, including the ability to monitor the cortical surface when inserting the rigid mandrel. Depression of the cortical surface can occur when advancing a blunt mandrel against an intact pial membrane. When the pia is penetrated, this runs the risk of hemorrhagic contusion associated with “brain rebound”. The utilization of a sharp mandrel when crossing the cortical surface minimizes the potential for this affect and has been effective to date. Crossing a sulci also produced a hemorrhage, which was initially contained but expanded after removal of the peel-away sheath. Trajectories are planned to avoid such a circumstance, but some instances are inescapable due to sulcal anatomy or intraoperative brain shift. Once detected, hemorrhage may or may not expand, and the removal of the peel-away sheath was found to be a critical stage for possible expansion of a hemorrhage. Accordingly, whenever hemorrhage has been detected we now perform repeat imaging as soon as reasonable after peel-away removal.CONCLUSION
Overall hemorrhage rates for MR guided DBS implantations were 2.4%/electrode implanted, with 1.1%/electrode being symptomatic. The findings are comparable to conventional surgical approaches and support the safety of MR guided DBS implantations.Acknowledgements
No acknowledgement found.References
[1] Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg. Mar 2010;112(3):479-490.
[2] Starr PA, Markun LC, Larson PS, Volz MM, Martin AJ, Ostrem JL. Interventional MRI-guided deep brain stimulation in pediatric dystonia: first experience with the ClearPoint system. J Neurosurg Pediatr. Oct 2014;14(4):400-408.
[3] Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg. Jan 2012;116(1):84-94.
Figures