5538
Interventional MRI at 3T: Compressed Sensing SEMAC for Improved Needle Visualization1The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Siemens Healthcare USA, 3Siemens Healthcare GmbH
Synopsis
Interventional MR imaging at 3 Tesla benefits from high signal and affords visualization and subsequent targeting of submillimeter structures, but needle artifacts may be exaggerated. Optimized fast gradient echo- and turbo spin echo-based pulse sequences minimize in-plane signal displacement, but through-plane artifacts remain. Compressed Sensing Slice-Encoding Metal Artifact Correction (SEMAC) MRI has the ability to minimize through-plane displacement, and thus holds promise to improve the accuracy of device localization. We demonstrate the clinical feasibility of Compressed Sensing SEMAC TSE for interventional MR imaging at 3 Tesla and visualization of the needle artifact with high accuracy.
Introduction
Interventional MR imaging at 3 Tesla benefits from high SNR and CNR and affords visualization and subsequent targeting of submillimeter structures such as small nerves [1]. However, a limitation of 3 Tesla can be the exaggeration of needle artifacts, which may impair the ability to precisely localize the needle’s tip relative to the target. Optimized fast gradient echo and turbo spin echo-(TSE)-based pulse sequences minimize in-plane signal displacement, but through-plane artifacts remain. Slice-encoding metal artifact correction (SEMAC) MRI has the ability to minimize through-plane displacement [2], and thus holds promise to improve the accuracy of device localization. However, the additional encoding steps for through-plane metal artifact correction result in longer acquisition times. Compressed sensing-(CS)-based data sampling strategies exploit the intrinsic sparsity of SEMAC imaging and can reduce the acquisition time by up to 60% [3].Purpose
To evaluate the feasibility and accuracy of compressed sensing Slice Encoding for Metal Artifact Correction (SEMAC) TSE for the visualization and targeting of MR-conditional needles during interventional MR imaging at 3 Tesla.Methods
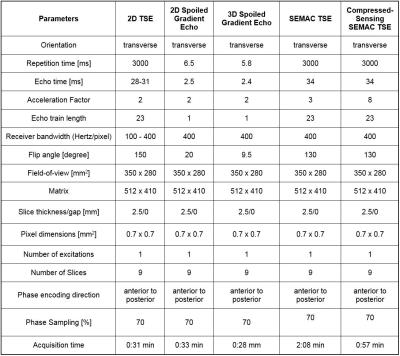
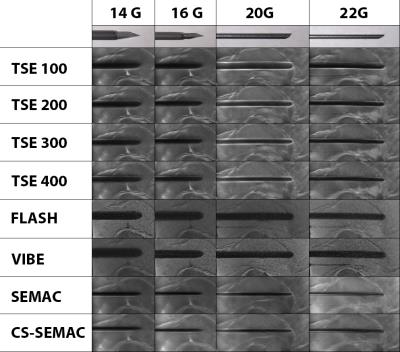
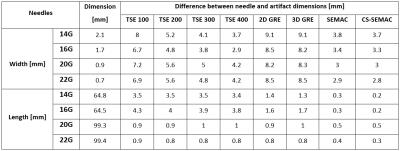
Institutional review board approval was obtained, and all subjects gave informed consent. A 3T MR imaging system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) was used in conjunction with a 4-channel receive-only flexible surface coil and the embedded spine array coil. We evaluated four commercially available MR-conditional needles of 14G (Invivo, Coax Needle), 16G (Invivo, Coax Needle), 20G (Cook, MReye Needle) and 22G (Cook, MREye Needle). Using an acrylic glass needle guide phantom, all needles were inserted into a pork shoulder orthogonal to the external static magnetic field, which mirrors our clinical practice. Eight pulse sequences including SEMAC TSE and a CS-SEMAC TSE prototype (Figure 1) were tested. Both SEMAC-based pulse sequences used 11 encoding steps. Conventional SEMAC used a 3-fold 1D GRAPPA acceleration, whereas 8-fold CS-SEMAC acceleration was achieved through incoherent undersampling of the 2D-phase encoding matrix and non-linear, SENSE-type reconstruction with L1-norm-based regularization [3] (Figure 1). Length and width of the needle artifacts were quantified using a full-width-half-maximum algorithm [4]. Five MR-guided percutaneous injection procedures were subsequently performed comparing TSE with a receiver bandwidth of 400 Hz/pixel and CS-SEMAC using the 20G (Cook, MReye) needle. The needle visualization was visually assessed by the interventionalist for both pulse sequences and subsequently ranked in accordance with operator preference.Results
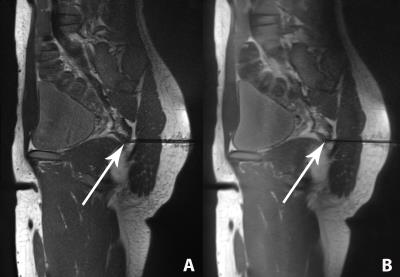
The needle was visualized by all pulse sequences with sufficient contrast to the surrounding muscle tissue (Figure 2). Artefact widths and lengths showed a marked dependency on the pulse sequence type and design (Figure 3). The widths and lengths of the needle artifact were largest on gradient echo images, and smallest and most accurate on SEMAC images. There was no significant difference between SEMAC and CS-SEMAC measurements. The needle tip location errors as indicated by the length of the needle artifacts was highest for the TSE pulse sequence with 100 Hz/pixel bandwidth and lowest for SEMAC and CS-SEMAC. All MR-guided procedures were successful with no complications. CS-SEMAC MR images better visualized the needle compared to 400 Hz/pixel bandwidth TSE in 4 out of 5 cases (80%) and similar in the other case. CS-SEMAC images additionally demonstrated a more favorable contrast of the dark needle artifact to surrounding muscle, ligament, tendons and fibrous tissues.Discussion
Our study demonstrates the feasibility of an 8-fold accelerated CS-SEMAC TSE pulse sequence for interventional MR imaging at 3 Tesla. CS-SEMAC TSE displays the needle artifact with the best accuracy and with a needle tip error of less than 1 mm. During interventional MR imaging procedures, the needle may be better visualized on CS-SEMAC images due to a more favorable contrast difference between the signal intensity of the needle and surrounding tissues. Due to the 2D phase-encoding scheme required for artifact reduction, the acquisition time of CS-SEMAC was about 1 min. CS-SEMAC may be most helpful for situations where the needle tip needs to be visualized with highest certainty, rather than routine use.Conclusion
CS-SEMAC TSE is feasible for interventional MR imaging at 3 Tesla and visualizes the needle artifact with high accuracy, which may be helpful for situations where the needle tip needs to be visualized with highest certainty.Acknowledgements
The authors would like to acknowledge Jens Wetzl and Michael Zenge for their work on the image reconstruction framework.References
1. Fritz J, Dellon AL, Williams EH, Belzberg AJ, Carrino JA. 3-Tesla High-Field Magnetic Resonance Neurography for Guiding Nerve Blocks and Its Role in Pain Management. Magn Reson Imaging Clin N Am. 2015 Nov;23(4):533-45.
2. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med. 2009 Jul; 62(1): 66–76.
3. Fritz J, Ahlawat S, Demehri S, Thawait GK, Raithel E, Gilson WD, Nittka M. Compressed Sensing SEMAC: 8-fold Accelerated High Resolution Metal Artifact Reduction MRI of Cobalt-Chromium Knee Arthroplasty Implants. Invest Radiol. 2016 Oct;51(10):666-76.
4. Thomas C, Wojtczyk H, Rempp H, Clasen S, Horger M, von Lassberg C, Fritz J, Claussen CD, Pereira PL. Carbon fibre and nitinol needles for MRI-guided interventions: first in vitro and in vivo application. Eur J Radiol. 2011 Sep;79(3):353-8.
Figures