5536
Accelerated 3D Echo Planar Spectroscopic Imaging of HIV: Metabolite Changes Correlation with CD4 count and Number of Years of Treatment1Human Magnetic Resonance Center, Institute for Applied Life Sciences (IALS), University of Massachusetts, Amherst, MA, United States, 2Radiological Sciences, University of California Los Angeles, Los Angeles, Los Angeles, CA, United States, 3Medicine, Harbor-UCLA Medical Center, Torrance, CA, United States
Synopsis
In vivo proton magnetic resonance spectroscopy studies of HIV-infected humans have demonstrated region-specific changes in brain metabolites including N-acetylaspartate, creatine, choline, glutamate/ glutamine, and myo-inositol. Using a 3D EPSI technique, we examined metabolite ratios with respect to creatine in several regions of brain in 18 HIV adults (mean age 46.2 years) and 15 healthy controls (mean age 43.4 years). We have demonstrated for the first time the feasibility of a novel accelerated 3D EPSI method in HIV-infected adults compared to age matched healthy controls and correlated with CD4 counts and number of years of treatment.
Introduction
Proton MRS of the brain is a non-invasive approach to probe cerebral biochemistry and is a sensitive method for detecting brain metabolites that reflect glial and neuronal changes. HIV infection causes glial activation and neuronal injury 1, 2. Myoinositol (mI) and choline (Cho) compounds are elevated in patients with HIV-associated chronic neuroinflammation and glial activation, while the neuronal marker N-acetylaspartate (NAA) is decreased in later or more severe stages of HIV associated dementia 3. In vivo MRS studies of HIV have demonstrated regionally specific changes in brain metabolites including NAA, creatine (Cr), Cho, glutamate/ glutamine, and mI. In this study we have implemented and validated an accelerated three dimensional (3D) echo planar spectroscopic imaging (EPSI) method utilizing a non-uniform sampling (NUS) scheme in combination with iterative, nonlinear reconstruction. This method was used to evaluate metabolic changes in HIV-infected adults compared to age matched healthy controls.Materials and Methods
18 HIV infected adults (mean age of 46.2 years) and 15 healthy controls (mean age of 43.4 years) were enrolled in this study. Inclusion criteria were: HIV infected males and females between the ages of 25 and 60 years, CD4+ count > 200 , HIV viral load < 50 copies/m. Exclusion criteria were: multiple sclerosis, brain neoplasms, hepatitis C co-infection and alcohol abuse/dependence. With the full encoding of one of 3 spatial and spectral dimensions enabled by the echo-planar bipolar gradients, a NUS scheme was applied to the phase encoding dimensions in order to accelerate the acquisition of 3D EPSI. Using this novel method, we examined metabolite ratios with respect to Cr in all subjects. The 3D EPSI parameters were: FOV = 240x240x120 mm3, image matrix = 32x32x8, spectral width = 1190 Hz, number of spectral points = 256, TE = 41ms, TR = 1.5s, Avg=12. SPSS 20 statistical software was used in the analysis and p-values less than or equal to 0.05 were considered significant.Results
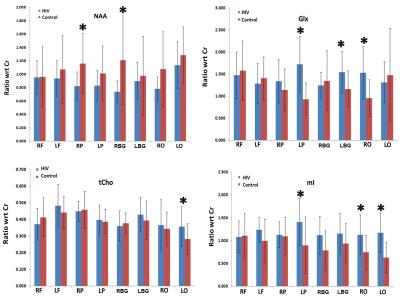
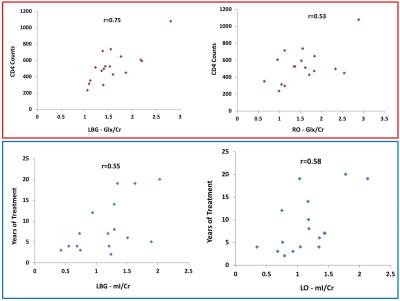
The mean metabolite ratios of the HIV+ participants and controls in multiple regions are shown in Fig.1. HIV patients showed significantly declined NAA/Cr in the right parietal (RP) (p=0.015), and right basal ganglia (RBG) (p=0.017) regions compared to controls. Also, a non-significant decline of NAA/Cr was observed in the right frontal white (RF) (p=0.954), left frontal white (LF) (p=0.417), left parietal (LP) (p=0.138), left basal ganglia (LBG) (p=0.644), right occipital (RO) (p=0.070) and left occipital (LO) (p=0.280) regions in the HIV+ participants. In addition, significant elevation of Glx/Cr was observed in the LP (p=0.001), LBG (p=0.018) and RO (p=0.003) regions of HIV+ participants compared to controls. Also, an elevation of Glx/Cr was observed in the RP (p=0.248) regions of HIV patients. However, a non-significant decline of Glx/Cr was observed in the RF (p=0.644), LF (p=0.507), RB (p=0.579) and LO (p=0.586) regions of HIV+ participants compared to controls. In the LO region, HIV+ participants showed significantly higher tCho/Cr (p=0.038) compared to controls. In addition, a non-significant elevation of tCho/Cr was observed in the LF (p=0.278), LP (p=0.724), LBG (p=0.447) and RO (p=0.763) regions of HIV+ participants. Also, decreased tCho/Cr was observed in the RF (p=0.313), RP (p=0.515), and RB (p=0.843) regions non-significantly. Statistically significant increases of mI/Cr were observed in the LP (p=0.017), RO (p=0.011), and LO (p=0.000) regions of HIV+ individuals compared to controls. Also, an elevation of mI/Cr was observed in the LF (p=0.121), RP (p=0.794), RBG (p=0.135), and LB (p=0.183) regions of HIV participants. Correlation coefficient between HIV patients, CD4 counts, years of treatment with metabolites were shown in the scatter plot (Fig.2).Discussion
Brain NAA levels are found to be reduced in a wide array of neurologic disorders and HIV 4,5 which agree with our results. Significantly increased Glx was observed in the LP, LBG and RO regions of HIV+ participants compared to controls. These findings are consistent with earlier reported associations between increased Glx and brain damage caused by chronic and acute hepatic encephalopathy, hypoxia, HIV and ornithine transcarbamylase deficiency 6, 7. If membrane damage happens following HIV infection, choline-containing compounds may be released and contribute to an elevated Cho signal. Higher concentration of inositol is found in pathological conditions such as Down’s syndrome, hypertonic stress and increased myelin breakdown in addition to gliosis which agree with current findings 8.Conclusion
The NUS-based CS reconstruction of multi-slice 3D EPSI will be useful for improved understanding of CNS dysfunction among HIV-infected individuals. This pilot study confirms evidence that there are neurometabolic differences between HIV adults and healthy controls using NUS compressed sensing based the accelerated 3D EPSI technique.Acknowledgements
NIH/NINDS: (#1R21NS086449-01A1).
References
1. Everall I, Luther P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol 1993; 52:561– 566.
2. Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol 1986; 19:525–535.
3. Chang L, Feger U, Ernst T. Bioimaging. In: Gendelman H, Grant I, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S (eds) The neurology of AIDS. Oxford University Press, New York, pp 2012; 763–797.
4. Richards TL. Proton MR spectroscopy in multiple sclerosis: value in establishing diagnosis, monitoring progression, and evaluating therapy. AJR Am J Roentgenol. 1991 Nov;157(5):1073-8.
5. Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010 Nov;32(5):1045-53.
6. Binesh N, Huda A, Thomas MA, Wyckoff N, Bugbee M, Han S, Rasgon N, Davanzo P, Sayre J, Guze B, Martin P, Fawzy F. Hepatic encephalopathy: a neurochemical, neuroanatomical, and neuropsychological study.J Appl Clin Med Phys. 2006 Winter;7(1):86-96.
7. Kreis R, Arcinue E, Ernst T, Shonk TK, Flores R, Ross BD. Hypoxic encephalopathy after near-drowning studied by quantitative 1H-magnetic resonance spectroscopy. J Clin Invest. 1996 Mar 1;97(5):1142-54.
8. Fisher SK, Novak JE, Agranoff BW. nositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002 Aug;82(4):736-54.