5515
High resolution MRSI using compartmental low rank algorithm: demonstration using undersampled EPSI1Department of Electrical and Computer Engineering, The University of Iowa, Iowa City, IA, United States, 2GE Healthcare, Postdam, Germany, 3GE Healthcare, Waukesha, WI, United States, 4GE Global Research, Munich, Germany
Synopsis
Improved spatial resolution is the need of the hour for MRSI. In this work we propose an algorithm which provides a comprehensive and automatic approach to recover high resolution metabolite maps from highly undersampled acquisitions; the improved spatial resolution translates to improved spectral quality and reduced leakage artifacts. The proposed algorithm is also quite flexible and can be readily used in a variety of sequences, including EPSI, CSI, and spirals acquisition schemes.
Purpose
Conventional MRSI schemes that rely on Nyquist sampling are fundamentally limited by the achievable spatial resolution. The low spatial resolution often results in B0 induced spectral distortions and spectral leakage from extracranial lipids and unsuppressed water, which makes the interpretation of MRSI data challenging. Lipid suppression schemes1-3 (e.g outer volume suppression, inversion recovery) have limited ability in eliminating the leakage artifacts, at the typically low spatial resolution of conventional MRSI. Novel acquisition schemes and reconstruction algorithms are urgently needed to overcome these challenges.Methods
We introduce a novel compartmental low rank algorithm, coupled with variable density undersampled k-space acquisition, to considerably improve the spatial resolution of the metabolites. The reconstruction from undersampled data is made well-posed by low-rank priors on the extracranial lipid and metabolite compartments, which are further constrained to be mutually orthogonal. The spatio-temporal MRSI signal is represented as the linear combination of the metabolite signal $$$\gamma_M$$$ and the lipid signal $$$\gamma_L$$$:
$$\gamma(\mathbf r,t) = \gamma_{\rm M}(\mathbf r,t)+\gamma_{\rm L}(\mathbf r,t),$$
where r and t are the spatial and temporal dimensions, respectively. We assume these signals to be spatially disjoint and restricted to the spatial regions $$$\Omega_M$$$ and $$$\Omega_L$$$, respectively. Since the spectral support and relaxation properties of the metabolites and lipids are strikingly different, they can be safely assumed to be mutually orthogonal (i.e, $$$\left\langle \gamma_M(\mathbf r_1,t),\gamma_L(\mathbf r_2,t)\right\rangle =0; \forall \mathbf r_1 \in \Omega_1; \mathbf r_2 \in \Omega_2$$$)4. We exploit this orthogonality property to minimize lipid leakage artifacts; we do not rely on elaborate spectral priors as in5,6, hence this method is more robust to field inhomogeneity variations in the extra-cranial regions. Since the recovery of the metabolites and lipids from highly under-sampled and heavily noisy data is highly ill-posed, we propose to use compartmental low-rank priors to make the problem well posed. We formulate the recovery as:
$$ \left\{\Gamma_{\rm M},\Gamma_{\rm L} \right\}= \arg \min_{\Gamma_{\rm M},\Gamma_{\rm L}}\underbrace{\|\mathcal{A}(\Gamma) - \mathbf b\|^2}_{\small\mbox{data consistency}} + \underbrace{\lambda_{1}\|\Gamma_{\rm M}\|_{*} ~+~\lambda_{2}\|\Gamma_{\rm L} \|_{*}}_{\small\mbox{low-rank priors}}+ \underbrace{\beta\| \Gamma_{\rm M} \Gamma_{\rm L}^H\|^2}_{\small \mbox{orthogonality prior}}$$
Here, $$$\mathcal A$$$ is the forward model consisting of inhomogeneity shifts, coil sensitivity encoding, and (non)-uniform Fourier transform, while $$$\mathbf b$$$ is the k-space data collected using EPSI. $$$\Gamma_{\rm L},\Gamma_{\rm M}$$$ are the Casorati matrices for the lipid and metabolite compartments respectively.
A volunteer was scanned with a GE MR750W 3T scanner at the University of Iowa using a 32-channel head coil. We relied on a hybrid EPSI acquisition scheme consisting of (a) a 32 X 32 sampled EPSI data with 8 averages for improved SNR and (b) a 96 x 96 sampled EPSI dataset with only one average. The readout bandwidth of both datasets is 600 Hz. The hybrid k-space data is constructed by retaining the low resolution part from (a) and only the high resolution part from (b). The total data acquisition time, including water reference acquisition for eddy current correction and sensitivity estimation, was 12 mins. We also considered undersampling the high-resolution data with factor x2 and x4 respectively which correspond to scan times of 9.5 mins and 8 mins. Water is removed using HSVD from the hybrid k-space data.
Results
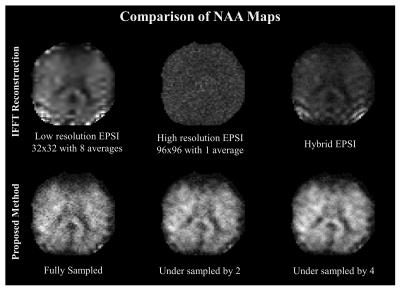
Data is collected on a single axial slice of FOV=24 cm, slice thickness of 1 cm at TR/TE = 800/100 ms. 8 OVS bands were used for lipid suppression. Figure 1 shows the peak integral images of NAA maps for IFFT reconstruction of the low resolution ( 32 X 32), high resolution (96 X 96) and the hybrid data in the top row. Bottom row shows the reconstruction using the proposed method for the fully sampled, and undersampled data. All maps are reconstructed to a grid size of 96 X 96. The IFFT reconstructions show lipid leakage near the skull for the low resolution and hybrid data whereas the SNR of the high resolution map is highly compromised. The proposed method produces high resolution maps with high quality details like low signal in ventricles. As is evident from the maps the high resolution details are intact even for undersampled recovery.
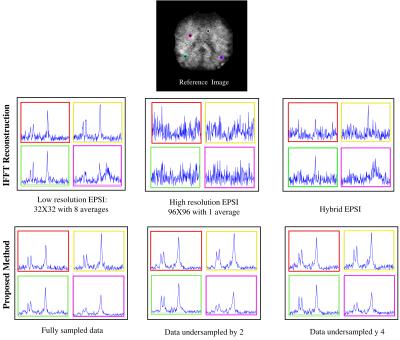
The spectra coresponding to the maps are shown in Figure 2. The low-resolution data and the hybrid data show lipid leakage in the pixels close to the skull whereas the high-resolution data has very low SNR. The representative spectra for the proposed method however, eliminate lipid leakage and are denoised.
Conclusion
We demonstrated a method for recovering high resolution MRSI data from undersampled measurements. The proposed method reduced lipid leakage artifacts and due to low rank modeling recovers high resolution maps from undersampled data. The maps can greatly improve with added spectral quantification.Acknowledgements
Grant Sponsors:
National Institute of Health, Grant number 1R01EB019961-01A1
National Science Foundation, Grant number F CCF-1116067,
American Cancer Society, Grant number RSG-11-267-01-CCE
Office of Naval Research, Grant number N00014-13-1-0202
References
[1] Chu, A., Alger, J. R., Moore, G. J., & Posse, S. (2003). Proton echo-planar spectroscopic imaging with highly effective outer volume suppression using combined presaturation and spatially selective echo dephasing. Magnetic resonance in medicine, 49(5), 817-821.
[2] Bydder, G. M., & Young, I. R. (1985). MR imaging: clinical use of the inversion recovery sequence. Journal of computer assisted tomography, 9(4), 659-675.
[3] Bottomley, P. A. (1987). Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences, 508(1), 333-348.
[4]Bilgic, Berkin, et al. "Lipid suppression in CSI with spatial priors and highly undersampled peripheral k-space." Magnetic resonance in medicine 69.6 (2013): 1501-1511.
[5]Lam, Fan, et al. "High-resolution 1H-MRSI of the brain using SPICE: Data acquisition and image reconstruction." Magnetic resonance in medicine (2015).
[6]Lam, Fan, and Zhi-Pei Liang. "A subspace approach to high-resolution spectroscopic imaging." Magnetic resonance in medicine 71.4 (2014): 1349-1357.
Figures