5511
Robust detection of 2-hydroxyglutarate at 7T high field with a fully adiabatic LASER sequenceMorteza Esmaeili1,2 and Ovidiu Cristian Andronesi1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, NTNU, Trondheim, Norway
Synopsis
With increasing number of high field 7T MR systems in clinical setting, the potential of using advanced MR modalities such as MR spectroscopy is increasing. Imaging 2-hydroxyglutarate (2-HG) can genotype IDH mutations in gliomas. However, the MR signal of this metabolite is overlapped with other resonances, hampering robust quantification of 2-HG. Here we optimized the timing of LASER sequence for robust detection of 2-HG at high field 7T in the presence of B1 inhomogeneity.
TARGET AUDIENCE
MRS sequence developers;
Neuroradiologists; Neuroscientists; Neurologists.PURPOSE
Accumulation of 2-HG metabolite in glioma patients that harbor isocitrate dehydrogenase (IDH) mutation is associated with improved clinical outcome and response to treatment1. Therefore, unambiguous and robust detection of this oncometabolite can benefit accurate gliomas stratification and targeted therapy. Despite recent development of MRS sequences2-4, improving sensitivity and specificity of 2-HG detection is still an area under investigation. Previous work using PRESS5 and semi-LASER4 sequences at 7T have shown that an inverted 2.25 peak can be obtained for 2HG which is distinguishable from modulation of glutamate and glutamine. However, spectral modulations are very sensitive to inaccuracies of flip angles which are exacerbated at 7T due to B1 inhomogeneity. In this work we aimed to optimize a fully adiabatic LASER sequence that can compensate better the B1 inhomogeneity for robust detection of 2-HG at high field 7T.METHODS
2HG, Glu, and Gln spectra were simulated for different echo timings of a LASER6 sequence at 7T field strength using GAMMA library. We analyzed the J-coupling modulation and the intensity of these metabolites for each sequence timing (Fig. 1). In particular our aim was to maximize the separation of 2.25ppm signal of 2HG from overlapping resonances. The LASER sequence consists of three pairs of adiabatic refocusing pulses, which can be separated in three sub-echo times TE1, TE2, and TE3, respectively. The best timing combinations for adiabatic refocusing RF pulses in the LASER sequence, i.e. TE1, TE2 and TE3 combination, were searched (Fig. 1) by simulations to provide a unique behavior of 2-HG resonances compared with other resonances, such as a negative intensity and increased amplitude of 2-HG signal with simultaneous decreased amplitude and opposite modulation of the neighboring metabolite resonances. In addition, we searched for the combinations that achieved these goals for the minimum total echo time. The candidate timing intervals in our LASER sequence were verified experimentally on phantoms containing 10 mM 2-HG and identical concentration of Glu and Gln, and 20 mM of glycine. In order to reduce specific absorption rate (SAR) of the LASER we used gradient modulated adiabatic pulses GOIA-W(16,4)6 of 5 ms duration and 20 kHz bandwidth that allowed us in vivo TR of 5s at 7T.RESULTS
A LASER sequence with TE=90 ms (TE1/TE2/TE3= 15/45/30 ms) provided a large negative 2-HG resonance at 2.25 ppm well separated from those of overlapped resonances. The same 2HG resonance pattern was observed in MR spectra obtained from phantoms applying the same timing intervals (Fig. 2), accompanied with reduced levels of overlapping peaks Glu and Gln (Fig. 3).DISCUSSION/CONCLUSION
The proposed sequence timing of our LASER sequence may provide unambiguous detection of 2-HG resonances on a 7T clinical scanner with high specificity and sensitivity. Our preliminary results from simulations and phantoms are currently under investigation in patients. Further, this technique provides a great opportunity to non-invasively monitor the effect of new anti-cancer drugs that target mutant IDH enzymes.Acknowledgements
No acknowledgement found.References
1. Yan, H. et al. IDH1 and IDH2 Mutations in Gliomas. New England Journal of Medicine 360, 765-773 (2009). 2. Choi, C. et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in subjects with IDH-mutated gliomas. Nature Medicine 18, 624-629 (2012). 3. Andronesi, O.C. et al. Treatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional Spectroscopic Mapping of 2-Hydroxyglutarate. Clinical Cancer Research 22, 1632-1641 (2016). 4. Emir, U.E. et al. Noninvasive Quantification of 2-Hydroxyglutarate in Human Gliomas with IDH1 and IDH2 Mutations. Cancer Res. 76, 43-49. (2016). 5. Ganji, S.K. et al. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magn Reson Med doi: 10.1002/mrm.26190 (2016). 6. Andronesi, O.C. et al. Spectroscopic imaging with improved gradient modulated constant adiabaticity pulses on high-field clinical scanners. Journal of Magnetic Resonance 203, 283-293 (2010).Figures

Figure
1. Simulations: heat map showing the amplitude of
metabolite signal modulation by simulation
of LASER pulse sequence with different inter-pulse timing. A windows of
15-100ms were used to implement different combination of inter-pulse delay.
Metabolites spin systems were separately generated for each time delays combination
using GAMMA library simulation. At the echo time of 90 ms (combination of TE1/TE2/TE3=15/45/30
ms), 2-HG exhibits large negative intensity while Glu and Gln were close to
zero based on the heat map.
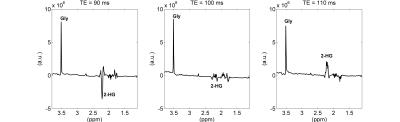
Figure
2. Experimental measurements: from left to right, 7T
LASER spectra with TE = 90 ms (TE1 = 15 ms, TE2 = 45 ms, and TE3 = 30 ms), TE =
100 ms (TE1 = 70 ms, TE2 = 15 ms, and TE3 = 15 ms) and TE = 110 ms (TE1 = 70
ms, TE2 = 20 ms, and TE3 = 30 ms) obtained from a phantom containing glycine (Gly, 20 mM) and
2-hydroxyglutarate (2-HG, 10 mM).

Figure
3: Experimental measurements: from left to right 7T
LASER spectra with TE = 90 ms (10 ms (TE1 = 15 ms, TE2 = 45 ms, and TE3 = 30
ms) and TE = 110 ms (TE1 = 70 ms, TE2 = 20 ms, and TE3 = 30 ms) obtained from a
phantom containing glycine (Gly, 20 mM),
glutamate (Glu, 10 mM) and glutamine (Gln, 10 mM).