5510
Optimal Echo Time for In-Vivo Glutamate Detection at 7T Using semi-LASER 1H-MRS1Medical Biophysics, The University of Western Ontario, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada
Synopsis
At ultra-high field (7T), the quantification of glutamate by 1H-MRS is more accurate and precise than at lower field strengths. The semi-LASER 1H-MRS pulse sequence has advantages at high field but requires the use of relatively long radio frequency pulses to reduce power deposition. Typically, the shortest achievable echo times (TE) are sub-optimal for glutamate detection. In this study, the optimal TE for glutamate detection was estimated by time-domain simulation and verified against in-vivo measurements. Using simulations, the optimal TE was found to be 125 ms. In-vivo measurements in one subject produced a result of ~102 ms. Both results suggest that the glutamate signal is greater at longer TEs (100-125 ms) when using semi-LASER at 7T compared to the shortest achievable TEs (40-60 ms).
Purpose
The J-coupled metabolite glutamate, measured by proton magnetic resonance spectroscopy (1H-MRS), has been shown to be altered in several neurological conditions including Alzheimer’s disease and epilepsy.1,2 Such metabolite changes are thought to precede structural alterations and may be valuable biomarkers for disease detection. At ultra-high magnetic fields (e.g. 4T, 7T), greater signal to noise ratio and spectral dispersion leads to better separation of the glutamate and glutamine resonances, increasing quantification accuracy and precision.3,4 However, high-field 1H-MRS suffers from increased chemical shift displacement error, B1 inhomogeneity, power deposition, and shorter T2* relaxation. The semi-LASER (localization by adiabatic selective refocusing) sequence5,6 overcomes several of these limitations by using high-bandwidth low-amplitude adiabatic full passage (AFP) refocusing pulses that are insensitive to B1 inhomogeneity.
To detect and measure J-coupled spin systems using 1H-MRS, the shortest achievable echo time is normally used to minimize signal modulation due to J-evolution and T2 relaxation. However, in the semi-LASER sequence, the adiabatic refocusing pulses must be made relatively long to stay within power deposition limits; typically requiring echo-times in the 38-50 ms range at 7T.7,8 This shortest achievable echo time is not optimal for glutamate detection. The purpose of this work was to determine the optimal echo time for glutamate detection at 7T using semi-LASER.
Methods
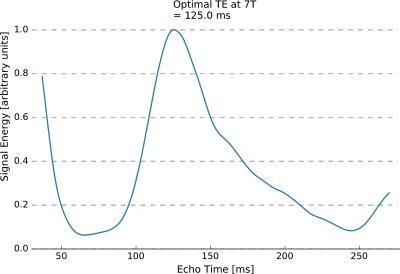
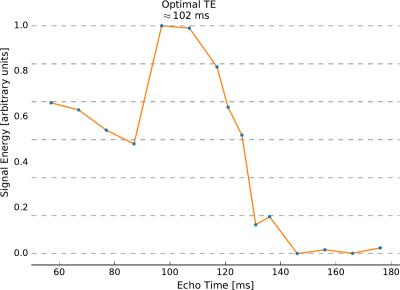
The optimal echo time for glutamate detection was estimated by time-domain simulation and verified against in-vivo measurements. Density-matrix simulations of glutamate at 7T were performed using the PyGAMMA software library.9 The radio frequency pulses of a semi-LASER sequence with a 4 ms asymmetric excitation pulse, 8 ms HS4-R25 AFP refocusing pulses,6 and echo times ranging from 37 ms to 270 ms (1 ms step size) were simulated. T2 effects10,11,12 were also modelled. Signal energies ($$$\int \left| FID(t) \right|^2 ~dt$$$) of the simulated time-domain glutamate signals were measured as an indicator of glutamate signal strength. These simulated results were compared to in-vivo measurements made on a 7T Siemens MAGNETOM head-only MRI using an 8-channel transmit and 31-channel receive coil array. In-vivo 1H-MRS spectra were collected from a single 2x2x2 cm3 voxel in the left primary motor cortex of one healthy individual, using the semi-LASER sequence (TR=7500 ms, 16 averages).5 Localized B0 and B1 shimming was performed for the voxel prior to data acquisition and VAPOR water suppression was optimized.13 Spectra were acquired at 15 different echo times ranging from 57 to 176 ms. The measured spectra were post-processed using combined QUALITY deconvolution and eddy current correction14 and fitted to TE-specific prior knowledge templates using the Levenberg-Marquardt minimization algorithm.15
Results
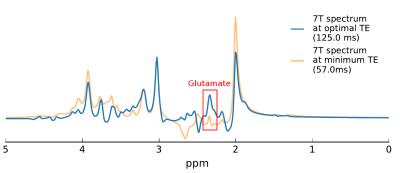
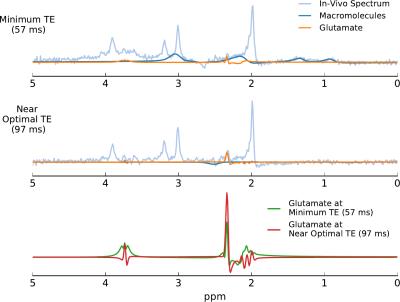
Using the density-matrix simulations, the optimal echo time for glutamate detection was found to be 125 ms (Figure 1). This echo time produced a larger glutamate signal energy than the shortest achievable echo time on our head-only system (57 ms). In-vivo measurements in one subject (Figure 2) produced an optimal echo time for glutamate detection of ~102 ms. Simulated spectra (Figure 3) at 57 ms and 125 ms demonstrate a 3-fold difference in glutamate multiplet signal at 2.3 ppm. In-vivo spectra (Figure 4) at 57 and 97 ms demonstrate a 2-fold difference in glutamate multiplet signal at 2.3 ppm.Discussion
Both the simulations and the in-vivo measurements suggest that glutamate signal is greater at longer echo times (100-125 ms) when using semi-LASER at 7T compared to the shortest achievable echo times (40-60 ms). Additional in-vivo measurements in multiple subjects and brain regions are needed to understand the small discrepancy in the optimal TE determined in-vivo and by simulation. Regardless, using a longer echo time has the added benefit of reducing signals from macromolecule resonances that complicate quantification at short echo times.Conclusion
The current practice in 1H-MRS is to use the shortest achievable echo-time to maximize the signal of J-coupled metabolites, including glutamate. However, this work has shown that this approach is sub-optimal for glutamate detection at 7T using semi-LASER, where echo-times below 40 ms are difficult to achieve. Instead, the optimal echo time for glutamate detection is in the range of 100 to 125 ms. Optimal glutamate detection with semi-LASER at 7T is important for the study of neurological conditions such as Alzheimer’s disease and epilepsy, in which glutamate plays a major role.Acknowledgements
Funding provided by the Alzheimer Foundation of London and Middlesex.References
1. Rupsingh, R., Borrie, M., Smith, M., Wells, J. L., & Bartha, R. (2011). Reduced hippocampal glutamate in Alzheimer disease. Neurobiology of Aging, 32(5), 802–10.
2. Peng, W. F., Ding, J., Mao, L. Y., Li, X., Liang, L., Chen, C. Z., … Wang, X. (2013). Increased ratio of glutamate/glutamine to creatine in the right hippocampus contributes to depressive symptoms in patients with epilepsy. Epilepsy and Behavior, 29(1), 144–149.
3. Bartha, R., Drost, D. J., Menon, R. S., & Williamson, P. C. (2000). Comparison of the quantification precision of human short echo time 1H spectroscopy at 1.5 and 4.0 Tesla. Magnetic Resonance in Medicine, 44(2), 185–192.
4. Tkác, I., Öz, G., Adriany, G., Ugurbil, K., & Gruetter, R. (2009). In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magnetic Resonance in Medicine, 62(4), 868–879.
5. Öz, G., & Tkác, I. (2011). Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magnetic Resonance in Medicine, 65(4), 901–910.
6. Garwood, M., & DelaBarre, L. (2001). The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. Journal of Magnetic Resonance (San Diego, Calif.: 1997), 153(2), 155–177.
7. Penner, J., & Bartha, R. (2014). Semi-LASER 1H MR spectroscopy at 7 Tesla in human brain: Metabolite quantification incorporating subject-specific macromolecule removal. Magnetic Resonance in Medicine, 12(1), 4–12.
8. Scheenen, T. W. J., Heerschap, A., & Klomp, D. W. J. (2008). Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. Magma (New York, N.Y.), 21(1–2), 95–101.
9. Smith, S. A., Levante, T. O., Meier, B. H., & Ernst, R. R. (1994). Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. Journal of Magnetic Resonance, Series A, 106(1), 75–105.
10. Marjanska, M., Auerbach, E. J., Valabrègue, R., Van de Moortele, P.-F., Adriany, G., & Garwood, M. (2012). Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR in Biomedicine, 25(2), 332–339.
11. Andreychenko, A., Klomp, D. W. J., de Graaf, R. A., Luijten, P. R., & Boer, V. O. (2013). In vivo GABA T2 determination with refocused echo time extension at 7T. NMR in Biomedicine, 26(11), 1596–1601.
12. Kreis, R., Slotboom, J., Hofmann, L., & Boesch, C. (2005). Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magnetic Resonance in Medicine, 54(4), 761–768.
13. Tkác, I., Starcuk, Z., Choi, I. Y., & Gruetter, R. (1999). In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magnetic Resonance in Medicine, 41(4), 649–656.
14. Bartha, R., Drost, D. J., Menon, R. S., & Williamson, P. C. (2000). Spectroscopic lineshape correction by QUECC: Combined QUALITY deconvolution and eddy current correction. Magnetic Resonance in Medicine, 44(4), 641–645.
15. Bartha, R., Drost, D. J., & Williamson, P. C. (1999). Factors affecting the quantification of short echo in-vivo 1H MR spectra: Prior knowledge, peak elimination, and filtering. NMR in Biomedicine, 12(4), 205–216.
Figures