5506
Ultra-short echo STEAM with TE of 3 ms improves 1H-MRS based lipid quantification and allows fast localization of 31P metabolite signals in the liver at 7T1High-field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 3Clinical Molecular Imaging, Karl Landsteiner Institute, Vienna, Austria, 4Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria
Synopsis
Liver fat quantification with in vivo 1H-MR spectroscopy is challenging at ultra-high fields also due to very short T2 times. Ultra-short TE localized sequence using Gaussian pulses with TE of 3ms was developed and its advantages could be utilized also with 31P-MR spectroscopy. Our data showed, that localization was feasible in large homogeneous tissues such as liver providing liver fat measurement with unprecedented precision. The detection of 31P signals was feasible and resulted in high spectral resolution in acceptable time.
Purpose
Proton (1H) and phosphorus (31P) MR spectroscopy play an important role in research of liver diseases enabling assessment of hepatocellular content of lipids (HCL)1 and changes in energy metabolism via ATP signals, glycogen synthesis via uridine diphosphoglucose (UDPG) and inflammation processes with assessment of nicotinamide adenine dinucleotide phosphate (NADPH)2. Knowledge about spin-spin (T2) relaxation is necessary for HCL quantification and its role is more pronounced at ultra-high fields (7T), where T2 of water in healthy liver was found to be 12 ms3. In addition, the liver iron content can decrease this already very short T2 time and the apparent relaxation behaviour of J-coupled systems is even more complex3,4. Advantages of ultra-short TE localized sequence could be employed also in 31P-MRS. It could prevent movement artefacts, which is a benefit over 3D-ISIS localization scheme5, provides shorter TE in comparison with semi-LASER6 (advantage for fast T2-relaxing metabolites, e.g. ATP7) and could take less acquisition time as 3D-CSI measurement8. However, achieving such a short TE is at the expense of well-performing RF pulses and spoiler gradients due to RF hardware and SAR limitations. The aim of this study was to develop a single-shot localized multinuclear sequence for 7T with the shortest TE possible employing Gaussian pulses. We focused mainly on assessment of the HCL and NADPH in the liver. As reference for the HCL assessment at 7T was used modified STEAM sequence - GUSTEAU3.
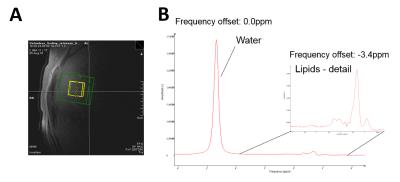
Methods
The original pulses from GUSTEAU3 sequence were replaced with Gaussian pulses (750µs), therefore termed G-GUSTEAU. The spoiler gradients were shortened to 460µs with a ramp-up-time of 300µs. The spoiler gradients strengths were 33 and 30 mT/m (TM spoiler gradient). These modifications resulted in a minimum TE of 3ms. All measurements were performed on whole body 7T scanner (Magnetom, Siemens Healthineers, Germany) using double tuned 1H/31P circular surface coil (diameter 10cm, Rapid Biomedical, Germany). Six healthy volunteers (age 30±3years, BMI 23±3kg.m-2, m/f=5/1) were studied for HCL with 1H-MRS and three for detection of 31P metabolites. 1H-MRS parameters: TE=3ms (G-GUSTEAU) and TE=6ms (GUSTEAU), TM=7ms, TR=5s, BW=3000Hz, NA=8, free breathing. The measurements were performed at frequency offsets of 0.0 (water) and -3.4ppm (CH2-lipids) to eliminate chemical shift displacement error (CSDE). Spectra were acquired without water suppression. Size of VOI was set to 2×2×2cm3 for G-GUSTEAU and 3×3×3cm3 for GUSTEAU. The 1H-MRS data were corrected with published T1 and T2 times3,4. The HCL was calculated according to formula: L/(W+L), where W represents the water and L represents the lipid signals (2.8, 2.2, 2.0, 1.3 and 0.9ppm). 31P-MRS measurements (TE=3ms, TM=7ms, TR=2s, BW=5000Hz, NA=64) were performed at frequency offsets of 0.0 (phosphocreatine) and -7.5ppm (α-ATP) to eliminate CSDE. Size of VOI was set to 4×4×3cm3. The phosphorus signals were quantified as ratio to the sum of 31P signals (α-ATP, NADPH, UDPG). Spectra were processed using the jMRUI with the AMARES9. In case of 1H-MRS, every single spectral transient for a specific TE was automatically frequency shifted, manually phased and summed before fitting. The last 200 points were truncated from the end of the FID and zero-filled back. No filter was applied before fitting.Results
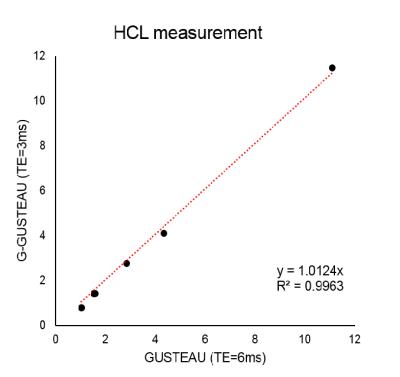
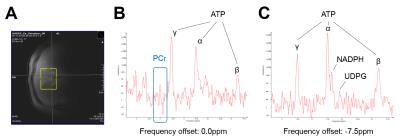
Placement of VOI in the liver tissue and 1H spectra measured with G-GUSTEAU are illustrated in Fig.1. HCL measurements with both sequences (with TE of 3 and 6ms) showed strong linear relationship (Fig.2). Placement of VOI for 31P-MRS and corresponding spectra are depicted in Fig.3. NADPH to γ-ATP ratio was 0.28±0.12 and to α-ATP was 0.29±0.06. The mean NADPH ratio was 0.20±0.03 and UDPG was 0.10±0.01.Discussion
Localization with Gussian pulse yields Gaussian-shaped (non-rectangular) volume. Thus, in order to avoid unwanted signal contaminations a smaller VOI has to be chosen and/or employed outer volume suppression scheme. The mean HCL error without T2 corrections using TE of 3ms was below 18%, however for precise HCL assessment, the T2 correction should be always applied. The detection of 31P signals was feasible without phosphocreatine contamination and resulted in high spectral resolution in acceptable time (2:08min per frequency offset). In case of measurements of metabolites with larger chemical shift differences, more frequency offsets have to be used, which is a limitation. Both ATP signals – γ and α – could be used for calculation of NADPH concentration. The 31P signal quantification can be further improved to provide absolute concentrations10.Conclusion
Localization with Gaussian pulses is feasible in large homogeneous tissues resulting in minimal TE of 3ms. G-GUSTEAU allows for HCL measurement of unprecedented precision and together with assessment of 31P metabolites makes this sequence a fairly simple and powerful multinuclear spectroscopic tool for advanced liver research.Acknowledgements
The authors wish to thank Dr. Vladimír Mlynárik, Dr. Martin Meyerspeer (Medical University of Vienna, Austria) and Dr. Ivan Tkác (University of Minnesota, USA) for helpful discussions and Anniversary Fund of Austrian National Bank (P15363) for financial support.References
1. Parente DB, Rodrigues RS, Paiva FF, et al. Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PLoS One. 2014 Nov 26;9(11):e112574.
2. Sevastianova K, Hakkarainen A, Kotronen A, et al. Nonalcoholic fatty liver disease: detection of elevated nicotinamide adenine dinucleotide phosphate with in vivo 3.0-T 31P MR spectroscopy with proton decoupling. Radiology. 2010 Aug;256(2):466-73.
3. Gajdošík M, Chadzynski GL, Hangel G, et al. Ultrashort-TE stimulated echo acquisition mode (STEAM) improves the quantification of lipids and fatty acid chain unsaturation in the human liver at 7 T. NMR Biomed. 2015 Oct;28(10):1283-93.
4. Gajdošík M, Chmelík M, Just-Kukurová, et al. In vivo relaxation behavior of liver compounds at 7 Tesla, measured by single-voxel proton MR spectroscopy. J Magn Reson Imaging. 2014 Dec;40(6):1365-74.
5. Bogner W, Chmelik M, Andronesi OC, et al. In vivo 31P spectroscopy by fully adiabatic extended image selected in vivo spectroscopy: a comparison between 3 T and 7 T. Magn Reson Med. 2011 Oct;66(4):923-30.
6. Meyerspeer M, Scheenen T, Schmid AI, et al. Semi-LASER localized dynamic 31P magnetic resonance spectroscopy in exercising muscle at ultra-high magnetic field. Magn Reson Med. 2011 May;65(5):1207-15.
7. Lei H, Zhu XH, Zhang XL, et al. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003 Feb;49(2):199-205.
8. Chmelik M, Považan M, Krššák M, et al. In vivo (31)P magnetic resonance spectroscopy of the human liver at 7 T: an initial experience. NMR Biomed. 2014 Apr;27(4):478-85.
9. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997; 129(1):35-43.
10. Pfleger L, Gajdošík M, Chmelík M, et al. Absolute quantification of hepatic metabolites using 3D localized 31P-MRS at 7T. ESMRMB 2016 33rd Annual Scientific Meeting, #218
Figures