5501
Homonuclear spectral editing to measure ectopic lipid composition in vivo with 1H-MRSLucas Lindeboom1,2 and Robin de Graaf3
1Dept. of Human Biology/Human Movement Sciences, NUTRIM school for Nutrition and Translational Research in Maastricht, Maastricht University Medical Center, Maastricht, Netherlands, 2Dept. of Radiology, NUTRIM school for Nutrition and Translational Research in Maastricht, Maastricht University Medical Center, Maastricht, Netherlands, 3Dept. of Radiology and Biomedical Imaging, Magnetic Resonance Research Center, Yale University School of Medicine, New Haven, CT, United States
Synopsis
1H-MRS has been used extensively to measure the total amount of lipids stored in organs like skeletal muscle and liver and it has been found that these so called ectopic fat stores are associated with insulin resistance. The role of the composition of these lipid stores (e.g. saturated vs. unsaturated fatty acids) in metabolic disturbances is unclear. Here we show the feasibility of spectral editing techniques to characterize lipid composition in adipose tissue and skeletal muscle in vivo. Estimations of lipid composition with our approach are in line with invasive biopsy studies.
Purpose
Previously it has been shown that increased storage of lipids in non-adipose tissue (i.e. skeletal muscle and liver) is associated with insulin resistance. Although there are indications that especially saturated fatty acids interfere with insulin signaling1,2, data on lipid composition in skeletal muscle and liver are scarce. The lack of non-invasive methodology might be an important reason that this topic is mainly overlooked. The spectral resolution of 1H-MRS at high field (7T) is sufficient to characterize the lipid composition in vivo3, but this approach is limited to adipose tissue and the ability to perform 1H-MRS at high field. Especially in skeletal muscle, the complexity of extramyocellular and intramyocellular lipid signals (EMCL and IMCL), will hamper accurate quantification of lipid composition. We here sought to use 1H-MRS homonuclear spectral editing to characterize the lipid stores in vivo at 4T.Methods
In this study we used a MEGA-sLASER4 sequence to exploit homonuclear scalar coupling within a fatty acid molecule. The principle of our approach is depicted schematically in figure 1. By using the scalar coupling between the methine resonance (resonance D, around 5.31 ppm) and the neighboring methylene resonances (resonance B and C, around 2.03 ppm and 2.77 ppm respectively), these latter resonances can be separated from any resonances obscuring these peaks in vivo. While both mono-unsaturated and poly-unsaturated fatty acids (MUFA and PUFA) are characterized by the appearance of resonance B, only the PUFA will also give a signal at 2.77 ppm (resonance C). The signal from the methyl group (resonance A, around 0.89 ppm) can be used as a reference for the total amount of fatty acids. We included 4 lean, healthy male subjects in this study (age between 31 and 45 years, BMI < 25 kg/m2). The protocol was approved by the local Institutional Review Board and informed consent was obtained from all participants before the study. All in vivo experiments were performed on a 4.0 T (170.4 MHz) Magnex magnet (Magnex Scientific Ltd, Oxford, UK) interfaced to a Bruker Avance Spectrometer (Bruker Instruments, Billerica, MA). Spectra were acquired from adipose tissue (voxel 20mm x 7mm x 40mm, 32 averages (NA)), soleus muscle (voxel 30mm x 20mm x 40mm, NA=768) and tibialis anterior muscle (voxel 18mm x 18mm x 40mm, NA = 768). TR was set to 1500 ms for all measurements. The offset of the frequence selective inversion pulses in the MEGA-sLASER were set to the methine resonance (+686 Hz relative to the bulk methylene resonance) in odd scans and was far off resonance (+2686 Hz) in even scans. Additional water suppression (VAPOR) was applied. Initial post-processing was performed in Matlab (phasing, frequency alignment and subtraction) and edited spectra were then analyzed in jMRUI, assuming Gaussian lineshapes.Results
In figure 2 typical examples of spectra acquired from the soleus muscle (SOL) and tibialis anterior muscle (TA) are shown. For qualitative comparison a short TE (12ms) STEAM spectrum is shown together with the edited spectra. In table 1 the calculated lipid composition is given. Please note that for the TA measurements, only results of 2 subjects were available.Discussion
Homonuclear spectral editing can be used to characterize ectopic lipid composition in vivo. The reported lipid composition in adipose tissue and skeletal muscle in this study is in line with previous invasive biopsy studies5,6. The use of this method in metabolic research will give insight into the role of ectopic lipid composition in the etiology of metabolic disease, such as type 2 diabetes (T2DM) and non alcoholic fatty liver disease (NAFLD).Acknowledgements
LL was supported by a Van Leersum Grant of the Royal Netherlands Academy of Arts (KNAW) and Sciences and a Albert Renold Travel Fellowship from the European Foundation for the study of Diabetes (EFSD).References
1. Dimopoulos et al. Biochem J (2006); 2. Manco et al. Metabolism (2000); 3. Ren et al. J Lipid Res (2008); 4. Andreychenko et al. MRM (2012); 5. Field et al. AJCN (1985); 6. Andersson et al. AJCN (2002).Figures

Figure 1.
Schematic representation of spectral editing approach.
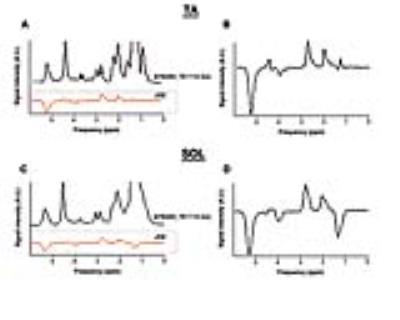
Figure 2. Typical
examples of edited spectra from TA and SOL muscle. A short TE STEAM spectrum is
shown for qualitative comparison.
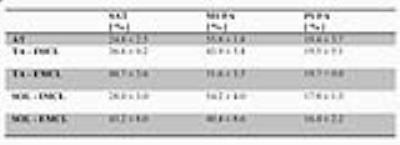
Table
1. Results from in vivo experiments in adipose tissue (AT), tibialis anterior muscle
(TA) and soleus muscle (SOL). Data are given as mean +/- SEM.