5492
Simultaneous Hadamard Editing of GABA and glutathione1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Biomedical Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Radiology, University of Calgary, Calgary, AB, Canada, 5Child and Adolescent Imaging Research Program, Alberta Children’s Hospital Research Institute, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Synopsis
Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) allows the simultaneous, separable editing of GABA and GSH, the two most frequently edited metabolites. Rather than a two-step ON/OFF encoding of
Purpose
Altered levels of GABA (γ-aminobutyric acid) and GSH (glutathione) are implicated in many neurological and psychiatric disorders(1,2). Although edited MRS of GABA is widely used(3), it is rarely combined in studies with the edited detection of other metabolites, such as GSH, because such a study would require multiple, long edited acquisitions. Here we describe Hadamard Encoding and Reconstruction of MEGA (MEscher-GArwood) Edited Spectroscopy (HERMES)(4) for the simultaneous and separable editing of GABA and GSH. We present phantom and in vivo measurements showing that multiplexed editing with HERMES effectively halves total scan time, compared to sequentially acquired GABA- and GSH-edited MEGA-PRESS acquisitions.Methods
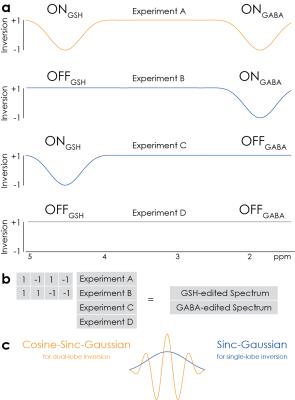
HERMES of GABA and GSH consists of four sub-experiments applying editing to both GABA and GSH (1.9 ppm for ONGABA, 4.56 ppm for ONGSH), GABA-only (ONGABA, OFFGSH), GSH-only (OFFGABA, ONGSH) or neither (OFFGABA, OFFGSH), as shown in Figure 1. The dual-lobe inversion pulse (Figure 1c) was generated by cosine-modulating a sinc-Gaussian editing waveform. The GABA-edited difference spectrum is calculated by subtracting the two OFFGABA scans from the sum of the two ONGABA scans, and the GSH-edited difference spectrum by subtracting the two OFFGSH scans from the ONGSH scans.
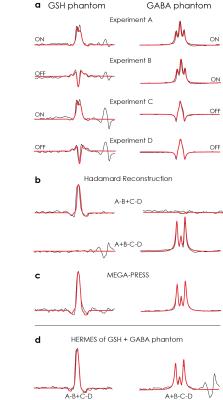
Phantom: Three phantoms were prepared in phosphate-buffered saline with: 4 mM GSH; 4 mM GSH and 4 mM GABA; and 10 mM GABA. In each phantom, three experiments were performed: HERMES; GABA-edited MEGA-PRESS; and GSH-edited MEGA-PRESS. Common acquisition parameters included: 27-ml voxel; TE/TR 80/2000 ms; 64 averages. The duration and full-width half-maximum bandwidth of the editing pulses were 20 ms and 62 Hz, respectively. Matched simulations of each experiment were performed in FID-A(5).
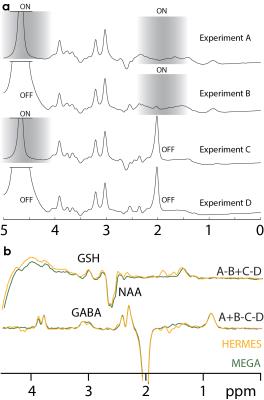
In vivo: 10 healthy adults were recruited under local IRB approval and gave informed consent. HERMES, GABA-MEGA- and GSH-MEGA-edited data were acquired from a single 46.7-mL voxel in the medial parietal lobe. All in vivo experiments were performed as for the phantom data, except with 320 averages. Each acquisition was ~11 minutes.
Post-processing: Data were filtered by a 3-Hz exponential, followed by frequency-and-phase correction using spectral registration in the time domain(6), as incorporated in Gannet(7). The averaged GSH-ON spectra were further aligned to the GSH-OFF spectra in the frequency domain, varying both frequency and phase to minimize the standard deviation of the choline subtraction artefact. Spectra were fit in Gannet and signal-to-noise ratios (SNR) calculated.
Results
Phantom acquisitions with overlaidDiscussion
Simulated, phantom and in vivo measurements of HERMES editing show excellent segregation of edited signals and excellent agreement with separately acquired MEGA-PRESS data. In practice, HERMES allows both GABA and GSH to be measured in the same duration and with equivalent SNR as a single MEGA-PRESS measurement. HERMES editing scheme now spans four TRs, rendering it more susceptible than MEGA-PRESS to subject motion and/or B0 field drift. As currently implemented, the protocol co-edits a substantial fraction of macromolecular signal, which could be eliminated by incorporating editing lobes at 1.5 ppm into the OFFGABA scans as in the macromolecular-suppressed GABA-edited experiment(8).Conclusion
HERMES has been demonstrated for GABA and GSH editing, allowing a two-fold acceleration of editing, while maintaining spectral quality, compared to sequentially acquired measurements using MEGA-PRESS.Acknowledgements
This work was supported by NIH grants R01 EB EB016089, R01 MH106564 and P41 EB015909.References
1. Foerster BR, Callaghan BC, Petrou M, Edden RA, Chenevert TL, Feldman EL. Decreased motor cortex γ-aminobutyric acid in amyotrophic lateral sclerosis. Neurology. 2012; 78(20):1596–600.
2. Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ,
3. Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA, Edden RAE. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014; 86:43–52.
4. Chan KL, Puts NAJ, Schär M, Barker PB, Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy: HERMES. Magn Reson Med. 2016; 76(1):11–9.
5. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit: The FID-A Toolkit for MRS Processing and Simulation. Magn Reson Med. 2015. DOI: 10.1002/mrm.26091.
6. Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015; 73(1):44–50.
7. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging JMRI. 2014; 40(6):1445–52.
8. Henry PG,
Figures