5491
Dual Voxel Diffusion Weighted MR-Spectroscopy1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 2Department of Applied Mathematics and Computer Science, Technical University of Denmark, Lyngby, Denmark, 3C. J. Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Centre, 4Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
Diffusion weighted spectroscopy is a technique with inherent long scan times. Here it was implemented in a simultaneous multi-voxel technique. This allows simultaneous assessment of multiple brain locations, and gives possibilities to extend the diffusion schemes with more directions and b-values.
Introduction:
Diffusion weighted spectroscopy (DWS) or diffusion tensor spectroscopy (DTS) is a promising technique [1] for probing cell specific microstructure with promising applications in MS [2]. However, the technique suffers from an intrinsically low sensitivity due signal attenuation from diffusion weighting and low concentrations of the target metabolites. The low SNR requires long scanning times for every diffusion direction and b-value. Therefore, scanning multiple locations sequentially, for example an additional contralateral control region, is limiting the number of b-values and directions that can be assessed. In this work we propose a simultaneous multi-voxel acquisition for DWS based on the ‘multi-band’ approach[3].
Methods:
Dual-voxel DW-MRS was performed at 7T to accelerate measurements with a factor of 2. Measurements were performed according to the local ethical protocols. Dual voxel DWS was performed based on the PRIAM [3] principle for parallel reconstruction in accelerated multi-voxel MR spectroscopy. This was performed by using a high bandwidth dual-band excitation pulse, which replaced the default 90˚ excitation pulse in the PRESS localization sequence. Separate left, right and simultaneous L+R voxels were collected using a 32-channel coil, and the separation of the signals was performed using the SENSE principles [4] based on the coil receiver sensitivity profiles, with a novel spatial offset correction along the frequency dimension to correct for the chemical displacements in the PRESS sequence. Experiments were performed in the human brain at 7T in the left and right splenium of corpus callosum; PRESS localization, cardiac triggering, TE=98ms 2x2x2cm3, 1 b=0 and 12 uniformly distributed directions with b=2300s/mm2 directions, 16 averages, scan time 15 min. DW-MRS data with and without water suppression was performed, phasing and eddy current correction was performed using the acquisition without water suppression. SENSE ‘g-factor’ values and ‘leakage factors’ [3] were to assess the noise level amplification and crosstalk between voxels. NAA diffusion tensor values were computed using linear regression and the principal directions were compared to those of a DTI acquisition (2x2x2mm3, 16 directions).
Results:
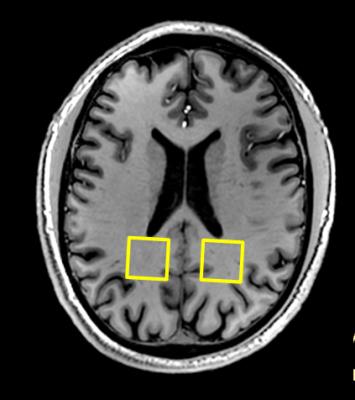
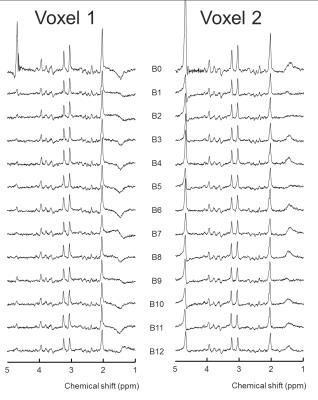
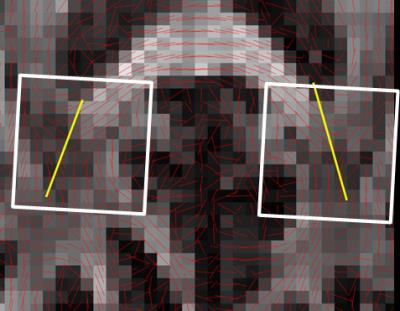
Unfolding of the simultaneously acquired voxels was possible at minimal SNR loss. The two voxel locations (figure 1) are unfolded with a g-factor of 1.0, meaning no noise amplification even though the voxels are quite close together. The leakage factor between the voxels was estimated at 1-2%. Unfolded spectra showed good quality although minor lipid distortions were observed (figure 2). The resulting directional information from the NAA signal corresponds well with the DTI data with the first eigenvectors following the extensions of the splenium of corpus callosum (figure 3).Discussion
Dual-voxel diffusion weighted MRS was possible at 7T in a diffusion weighted PRESS sequence with a dual-band excitation pulse. Although the dual-band pulse shows a reduced bandwidth, the loss of bandwidth in the excitation pulse was not more than that in the refocusing directions, and was accounted for in the unfolding algorithm. The method can in principle be extended to more voxel locations, extended to more than 1 dimension or be combined with MRSI to accelerate further. Compared to interleaving 2 voxels within 1 TR [5], the current method is truly simultaneous, has a negligible SAR increase (~1%) and provides more freedom in voxel placement.Acknowledgements
Danish Council for Independent Research (4093-00280A, 4093-00280B and 6111-00349A)References
1. Ronen I, Ercan E, Webb A. Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. 2013;7:13
2. Wood ET, Ronen I, Techawiboonwong A, Jones CK, Barker PB, Calabresi P, Harrison D, Reich DS. Investigating axonal damage in multiple sclerosis by diffusion tensor spectroscopy. 2012;32(19):6665
3. Boer VO, Klomp DW, Laterra J, Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn Reson Med. 2015;74(3):599
4. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;43(5):952
5. Ernst T, Hennig J. Double-volume 1H spectroscopy with interleaved acquisitions using tilted gradients. Magn Reson Med. 1991;20(1):27
Figures