5486
Repeatability of myocardial creatine and triglyceride concentration measurements with 1H-MRS1Department of Radiology, Academic Medical Center, Amsterdam, Netherlands, 2C.J. Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Department of Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands, 4Department of Cardiology, Academic Medical Center, Amsterdam, Netherlands, 5Neuroimaging Center, University Medical Center Groningen, Groningen, Netherlands
Synopsis
Changes in myocardial total creatine (tCr) and triglyceride (TG) content have been linked to the pathogenesis of cardiomyopathy. An assessment of repeatability for absolute quantification of myocardial [tCr] and [TG] is required for 1H-MRS to be employed for longitudinal monitoring of therapeutic efficacy. This work reports intra- and inter-exam repeatability of [tCr] and [TG] measurements with 1H-MRS at 3 Tesla. Our results indicate that the repeatability of the 1H-MRS assay will be sufficient to detect a >50% depletion of the tCr pool, and >50% changes in myocardial TG levels with 95% confidence in a single subject.
Purpose
This work aims to assess intra- and inter-exam repeatability of in vivo total creatine [tCr] and triglyceride [TG] concentration measurements in human myocardium using proton MR spectroscopy (1H-MRS) at 3 Tesla.
Background
Changes in myocardial total creatine (the sum of free creatine and phosphorylated creatine) and triglyceride content have been linked to cardiac pathology such as heart failure (creatine depletion in dilated cardiomyopathy)1,2 and diabetes mellitus (cardiac steatosis due to triglyceride accumulation)3. Although technologically challenging, noninvasive detection of these metabolites in the human heart is possible with 1H-MRS. Even though MRS is a quantitative technique, only few MR studies have estimated the absolute concentrations of myocardial tCr1,4,5 and TG6. An assessment of repeatability for absolute quantification (in mmol L-1 water; mM) of myocardial [tCr] and [TG] is an important prerequisite for 1H-MRS to be employed in clinical investigations, and for comparisons of MR results with other assays. Furthermore, it would greatly benefit computational model parameterization and validation in predictive medicine.2 Here, we report the intra- and inter-exam repeatability of in vivo 1H-MRS measurements at 3 Tesla tailored to absolute quantification of human myocardial [tCr] and [TG].
Methods
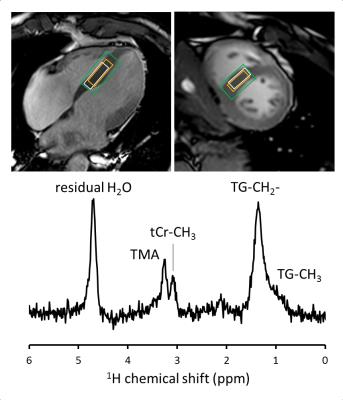
1H-MRS: All scans were performed on a clinical 3 Tesla MR system (Ingenia; Philips Healthcare, Best, The Netherlands), equipped with a quadrature body coil for RF transmission, and 12-element (posterior) and 16-element (anterior) coil arrays for signal reception. An ECG-triggered and respiratory-gated point resolved spectroscopy (PRESS) sequence was used for localized 1H-MRS of the interventricular septum.7 A high-permittivity pad8 was placed over the heart to improve transmit efficiency and receive sensitivity in the volume of interest. General sequence parameters: voxel size 10 × 25 × 30 mm (Figure 1); pencil beam B0 shimming; navigator gating window 3 mm; trigger delay 200 ms after ECG R-wave detection; TE 40 ms; 1024 data points; band width 1500 Hz. MOIST water-suppressed spectra: 64 averages; TG (1.3 ppm) on-resonance; TR 6 seconds + next heartbeat. Water spectra: 8 averages; water (4.7 ppm) on-resonance; TR 9 seconds + next heartbeat. Acquisition time: ~16 minutes. To assess method repeatability, 16 volunteers (8 male/8 female; age 29.4 ± 3.2 y; body mass index 22.5 ± 1.9 kg m-2) were scanned three times within 90 minutes: twice during the same magnet session (intra-exam repeatability) and once after exiting and re-entering the magnet (inter-exam repeatability).
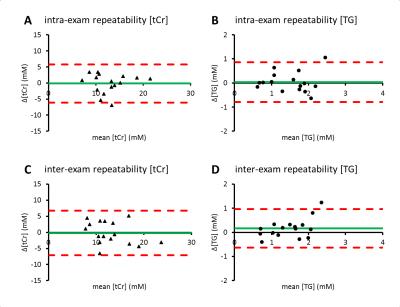
Data analyses: Signals from individual channels were phased, combined, eddy-current and DC-offset corrected and averaged using MATLAB (MathWorks, Natick, MA) to obtain a single spectrum for signal quantification. Spectra were fitted in the time domain using AMARES in jMRUI. The unsuppressed water signal and the creatine-methyl signal at 3.02 ppm were fitted to Lorentzian line shapes. The lipid signals at 0.85 - 1.55 ppm were fitted to four Gaussian line shapes. Myocardial [tCr] and [TG] were calculated after T2 relaxation9 correction of water (31 ms), creatine-methyl (135 ms), and TG-methylene (-CH2- at 1.3 ppm, 89 ms) signal amplitudes, and assuming 2 protons/water, 3 protons/creatine-methyl, and 68 protons/TG-methylene,6 and a water concentration reference of 55.5 mol L-1 water. Because spectra were acquired under fully relaxed conditions, no T1 relaxation corrections were required. Bland-Altman analyses were performed to determine intra- and inter-exam repeatability of [tCr] and [TG], with the repeatability coefficient defined as 1.96 × the standard deviation (SD) of the difference between two measurements in the same subject.
Results
Figure 1 shows a typical 1H-MR spectrum acquired from the interventricular septum of a healthy volunteer. Measured in 16 subjects, estimates (mean ± SD) of myocardial [tCr] and [TG] were 12.8 ± 3.7 mM and 1.5 ± 0.6 mM, respectively. The intra-exam [tCr] repeatability coefficient was 6.0 mM (46.5%) with a mean difference of -0.2 mM, and 0.8 mM (55.1%) with a 0.0 mM mean difference for [TG] (Figure 2A-B). Inter-exam repeatability coefficients were similar: 7.0 mM (53.6%) with a -0.2 mM mean difference for [tCr], and 0.8 mM (53.7%) with a mean difference of 0.2 mM for [TG] (Figure 2C-D).
Discussion
Intra- and inter-exam repeatability of myocardial [tCr] and [TG] measurements with 1H-MRS under fully relaxed conditions at 3 Tesla will be sufficient to detect a >50% depletion of the total creatine pool in cardiomyopathy1, and >50% changes in myocardial lipid levels3 with 95% confidence in a single subject. The estimate of human myocardial [tCr] found here is considerably lower than in previous studies (29 mM5 - 42 mM1,4), and is in closer agreement with biochemical assays of [tCr] in human myocardium (18 mM)10.
Acknowledgements
This work was supported by the National Institutes of Health (NIH grant HL072011).
References
1. Nakae I, et al. J Am Coll Cardiol 2003;42:1587-93
2. Tewari SG, et al. J Mol Cell Cardiol 2016;94:162-75
3. McGavock JM, et al. Circulation 2007;116:1170-75
4. Bottomley PA, Weiss RG. Lancet 1998;351:714-18
5. Weiss K, et al. Magn Reson Med 2012;68:1696-1704
6. Szczepaniak LS, et al. Magn Reson Med 2003;49:417-23
7. de Heer P, et al. J Magn Reson Imaging 2016;44:1151-58
8. de Heer P, et al. Invest Radiol 2015;51:134-8
9. Krssák M, et al. MAGMA 2004;16:155-59
10. Cowan DW. Am Heart J 1934;9:378-85
Figures