5464
A web-based searchable system to confirm MRI compatibility for medical implants in Japan1Department of Medical Imaging, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan, 2Medie Corporation, Tokyo, Japan
Synopsis
The purpose of this study was to develop a web-based searchable MRI safety information system for confirming medical implant compatibility and to evaluate the usefulness of the system. The system allows MRI compatibility confirmation to be performed over internet. This system facilitates obtaining MRI safety information for medical implants easily and rapidly, thereby improving the safety of MRI examination.
PURPOSE
It is mandatory to confirm the MRI compatibility of medical devices implanted in patients before conducting MRI examinations.1 In Japan, there are very few confirmation methods in use, and these are time consuming. We hypothesize that offering MRI safety information related to implants will reduce the time required for confirmation and will improve the safety of MRI examinations for patients. The purpose of this study was to develop a web-based searchable MRI safety information system for confirming medical implant compatibility and to evaluate the usefulness of the system.METHODS
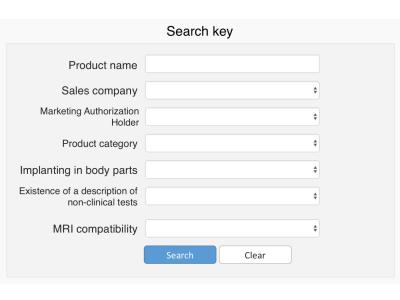
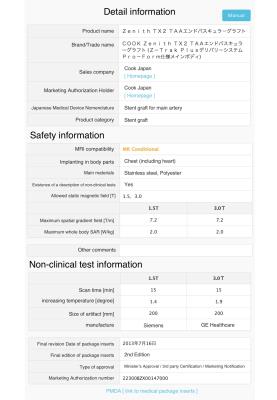
First, MRI compatibility of vascular stents and stent-grafts that had been sold in Japan as a sample (approximately 100 devices) was investigated and an MRI compatibility database was constructed. The MRI compatibility of the devices was determined by interviewing 20 manufacturers and based on their medical package inserts. The items investigated were as follows: 1. product name, 2. body parts for implanting and main material of the product, 3. whether medical package inserts include a description of MRI compatibility conducted, 4. MRI compatibility and conditions (maximum allowed static magnetic field, spatial gradient field and specific absorption rate), 5. conditions of non-clinical tests (increasing temperature, artifact size and MRI vendor), 6. medical package inserts and its final revision date. These information were collected and stored in a Microsoft Excel workbook. An MRI compatibility database was created based on the previously proposed lists for MRI labeling information.2,3 We classified the devices into five MRI compatibility status groups based on their medical package inserts as follows: 1. MR Safe, 2. MR Conditional, 3. MR Unsafe, 4. Unknown (which has no description of non-clinical test based on ASTM), 5. No medical package inserts. Finally, a web-based searchable system was developed to use the MRI compatibility database (Fig.1). A total of 400 medical staff and MRI vendors participated in evaluating the system. The participants evaluated the usefulness of the system based on a questionnaire. The study protocol was approved by the Institutional Review Board of Kumamoto University.RESULTS AND DISCUSSIONS
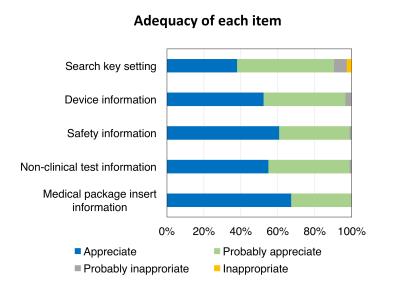
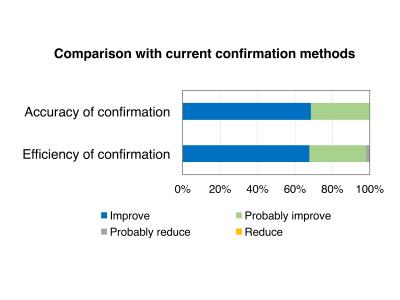
The MRI compatibility information of 79 devices was obtained from 12 manufacturers (approximately 80% of the vascular stents and stent-grafts sold in Japan). The system allows MRI compatibility confirmation to be performed over internet. Figures 2 and 3 show a display of the search key setting and search result, respectively. 264 responses were received for the questionnaire with a response rate of 66%. The medical staff confirmed the existing use of several methods, based on, for example, medical package inserts, date of implant or device type, interviewing device supplier, or the website of MRIsafety.com.4 However, the aggregated MRI compatibility information is still needed by the medical staff. Figures 4 and 5 show the evaluation results by the medical staff for this system. Most reviewers answered that the search key setting and indication methods of the detailed MR compatibility information in this system were appreciable. Moreover, they evaluated that the accuracy and efficiency of confirmation can be improved using this system compared with traditional confirmation methods. The database was created in a consolidated format based on medical package inserts. It is thus easy to obtain MRI compatibility information for devices implanted in patients. Moreover, this system may improve the quality of MRI safety management for patients. However, the database currently contains a limited number of devices and categories. In the future, the database must be updated by collecting data from other manufacturers and devices.CONCLUSION
This system facilitates obtaining MRI safety information for medical implants easily and rapidly, thereby improving the safety of MRI examination.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 15K21240.References
1. Sawyer-Glover AM, Shellock FG. Pre-MRI procedure screening: recommendations and safety considerations for biomedical implants and devices. JMRI. 2000;12:92–106.
2. Shellock FG, Woods TO, Crues JV. MR labeling information for implants and devices: explanation of terminology. Radiology 2009;253:26–30.
3. K Kuroda, et al., Research report of contract research for Ministry of Health, Labor and Welfare 2015.
4. Shellock FG. MRISAFETY.COM. http://www.mrisafety.com Access April, 14, 2016.
Figures