Xiaoyi Min1 and Shiloh Sison2
1St. Jude Medical, Inc, Sylmar, CA, United States, 2St. Jude Medical, Inc, Sunnyvale, CA, United States
Synopsis
The objective of this study is to compare transfer
functions (TFs) of a Spinal Cord Stimulation (SCS) system in high permittivity
media (HPM), low permittivity media (LPM) and in spinal cord by using computer
simulations. For a SC model, we modeled
the anatomical and conductive properties of the lower thoracic (T7-T10) spinal
cord. The
TF curves in HPM and in SCS follow each other closely. With a SC model, the compound tissue effect around
the lead was seen. Lowering HPM
conductivity would shift the curve slightly up that
with the SC model for in-vivo.
Introduction
Spinal cord stimulation (SCS) is an established therapy for
treatment of chronic pain of the back and limbs. The SCS lead is placed in the dorsal epidural
space and is connected to an implanted pulse generator (IPG) placed in a
subcutaneous location that delivers electrical current through the lead to the
spinal cord. RF-induced heating during
an MRI scan can pose a risk to patients implanted with a SCS device. The amount
of RF-induced lead heating is mainly attributed to the electric field tangential
(Etan) to the lead path, which can be predicted by using an electrical field
transfer function (TF) associated in different media. The ISO/TS 10974 RF heating methodology
states that both HPM and LPM should be evaluated for SCS systems since more
than 10% of the lead length are tunneled subcutaneously. The objective of this study is to compare
electric field transfer functions (TFs) of a SCS system in high permittivity media
(HPM), low permittivity media (LPM) and in mixed media spinal cord (SC) by
using computer simulations.Methods
The ANSYS HFSS software package was used
to calculate the electromagnetic field distribution in a model built from the
TF measurement set-up. A St. Jude Medical Orion IPG and a percutaneous
Octrode lead model with lead length of 60 cm was built inside a tank (120 cm by
15 cm by 10 cm). The tank is filled with homogenous media such
as global average (HPM) (σ=0.47 S/m; ε =78) from
ASTM F2182 or fat tissue (LPM) (σ=0.0531 S/m; ε =11.5) from
ISO/TS 10974. For the mixed media of SC, we modeled the
anatomical and conductive properties of the lower thoracic (T7-T10) spinal
cord. Additionally, the lead was placed
symmetrically over the spinal cord cerebrospinal fluid (CSF) layer (σ=2.066
S/m; ε =97.3) within the dorsal epidural space
(σ= 0.04 S/m, ε =6.5)
with lead position varied dorsally. The
lumens of the lead were filled with either air (dry) or fluid (wet). The reciprocal
method was used with current excitation was at the distal electrode. Currents along lead conductors are obtained
by loop integrals of H fields at 1 cm increments starting from the distal
electrode. The TFs are the currents
along the lead divided by the excitation, denoted as S(τ). The
RF heating can be estimated1 by using ΔT = |ΣEtan(τ) *S(τ) dτ | and the theoretical worst
case is ΔT = A|Σ
|Etan(τ)| *|S(τ)| d t |2 when Etan is conjugate of S, where A is
a coefficient.Results
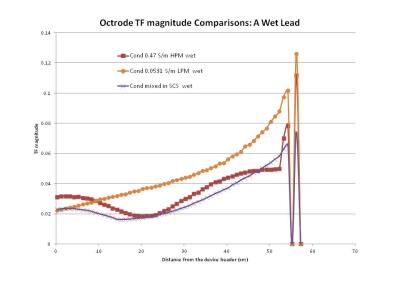
The curves of TF magnitudes |S(τ)| for HPM and mixed in SCS follow each other closely,
while the TF shape and scaling in LPM is significantly
different. The case with a wet lead is shown in Figure 1.
Conclusions
The results indicate that SCS TF
measurement and validation should be performed in HPM since it has the closest
match with mixed media SC. With the SC mixed media model, the compound tissue
effect from epidural fat and CSF around the lead was seen and the TF follows that
of the HPM TFs closely. Lowering HCM
conductivity would shift the curve slightly above that with the SC model for in-vivo
in order to ensure a worse case. This
can be further validated through DT
calculation of TF equation by integrals of Etan along clinical SCS implant
pathways for in-vivo temperature distribution estimation.
Acknowledgements
No acknowledgement found.References
1: Park
SM, Kamondetdacha
R, Nyenhuis
JA. Calculation of MRI-induced heating of an implanted medical lead wire with an
electric field transfer function. J Magn Reson
Imaging. 2007 Nov;26(5):1278-85.