5460
fMRI in Parkinson's Disease: Post STN-DBS implant.1Neuromodulation Center, Case Western Reserve University, Cleveland, OH, United States, 2Neurology, University Hospitals, Cleveland Medical Center, Cleveland, OH, United States
Synopsis
We present a method to conduct fMRI in Parkinson’s disease patients with fully implanted bilateral STN Medtronic Activa DBS. This allows for fMRI evaluation of clinically optimized settings or other settings that may reveal anatomical correlations with benefits and side effects.
Purpose
The permanent damage to implantable pulse generator (IPG) or heating of DBS leads are the potential risks involved with MR imaging in Sub-thalamic Deep Brain Stimulation (STN-DBS) implanted Parkinson's disease patients due to RF deposition by high specific absorption rate (SAR) MRI sequences. The adverse effects in conducting MRI in DBS implanted Parkinson's patients limits the clinician's needs to review the effects of STN-DBS on functioning of basal ganglia and cortical circuitry1,2.Introduction
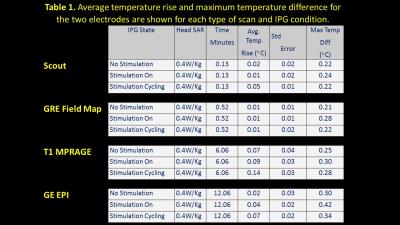
A preliminary study conducted with Medtronic DBS leads (3389) and IPG (Activa PC 37601) using a phantom determined DBS safety within the MR environment and during scans at Case Center for Imaging Research (CCIR). Overall, no measurements were observed that could compromise patient safety with temperature values consistently below 0.5°C, and with an average well below the accepted maximal limit of 1°C (details in Fig 1). Although human fMRI has been safely conducted earlier 5,6 our study is novel in two ways:
(1) Use of actual IPG and electrodes in a PD patient as compared to using lead extensions 6 running through the MR room.
(2) Evaluation of fMRI activation recorded in response to the therapeutic and sub-therapeutic settings of the IPG.
Methods
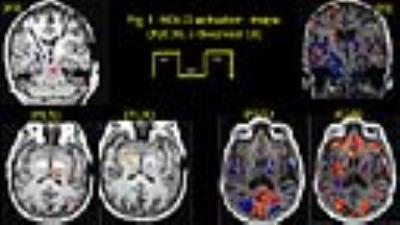
We ensured (a) temperatures ≤ 1°C at the lead contacts (b) intact lead contacts using DBS impedance studies, (c) regular IPG cycles within the scanner for the low SAR sequences used, and using (d) custom built Tx/Rx Head coils (e) chest implanted newer IPG with built in safety shunt circuitry (without magnetic reed switch). Two right-handed fully implanted bilateral STN-DBS PD patients were observed at their individual therapeutic and sub-therapeutic DBS settings (Medtronic DBS leads 3389) and IPG (Activa PC 37601). Imaging (“on-medication”) was performed at CCIR on a 3T Verio (Siemens Healthcare, Erlangen, Germany) with a custom built head coil (courtesy: Quality ElectroDynamics, Cleveland,(OH) USA). Isovoxel T1 (1mm3) MPRAGE was acquired in sagittal plane for anatomical reference and lead localization with 176 axial slices, 256x256, FOV=256x256mm, TR/TE/TI/α: 1900/1.83/900ms/9°. Fieldmaps were used to correct magnetic susceptibility artefacts using GRE sequence with TR/TE(1)/TE(2)= 390/4.89/7.35 ms, FOV 256, 32 slices 4 mm; 64x64 matrix, α= 60°. With IPG cycling at 30s, 120 volumes (30 slices/4mm each) of single shot EPI images were acquired with IPG cycling (30s on/off) in transverse slices in AC-PC plane with the following parameters: TR/TE 3000/29, FOV 256, 128x128; α: 80° for both left and right electrode separately at therapeutic and sub-therapeutic DBS settings (details in Fig 2). Data was processed offline using FSL (ver 5.0). The fMRI results were registered to the post-operative native T1 resliced to match the angle of the electrode.Results
Significant BOLD activation were observed in following regions:
Left electrode: Therapeutically, both patients evoked BOLD activation in bilateral occipital cortex, left thalamus, and contralateral cerebellum, P2 presented activation in globus pallidus interna (GPi) also; while sub-therapeutically, P1 presented similar activation as therapeutic, but P2 failed to present activation in left thalamus and pallidum.
Right electrode: Therapeutically, P1 presented a clear significant BOLD activation in pallidum, P2 presented no activation in pallidum or thalamus; while sub-therapeutically, P1 presented similar activation as for therapeutic, P2 did not evoke any activation in thalamus or pallidum.
Discussion
The activation around the electrode and pallidum during therapeutic stimulation of right electrode in P1, and that of thalamus and GPi left electrode therapeutic stimulation in P2 suggests that fMRI can detect effective DBS stimulation. Similar activation in P1 for both therapeutic and sub-therapeutic stimulation suggests that possibly a lower voltage could presumably produce the same result. It should be noted that patients in this study were scanned in the “on-medication” state with the DBS cycling on and off every 30 seconds for the box-car fMRI design. This was chosen to show the short term effects of DBs to understand the areas of tissue activation and their distant direct connections depending on unique lead position, patient anatomy and stimulation settings. Other study designs performed in “off-medication” state with DBS either chronically “on” or “off” are necessary to understand areas of brain activation that correlate with symptomatic benefit from long-term stimulation which may result from both short latency effects as well and long term network changes.Conclusion
This pilot study sample is too small to conclude any clear hypotheses from fMRI activation pattern, but it clearly indicates a safe fMRI acquisition for post STN-DBS implanted patients. This study opens a new direction for evaluation of patient specific therapeutic voltage threshold, apart from establishing the erroneous activation responsible for side effects in post STN-DBS implanted patients.Acknowledgements
Case Center for Imaging Research, Cleveland Medical Center, Cleveland (OH), USA
Neuromodulation Center, School of Medicine, Cleveland Medical Center, Cleveland (OH), USA
Department of Neurology, University Hospitals, Cleveland Medical Center, Cleveland (OH), USA
References
1. Henderson JM, Tkach J, Philips M, et al.(2005) Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for parkinson’s disease: case report. Neurosurgery 57:E1063.
2. Spiegel J, Fuss G, Martin Backens, et al. (2003) Transient dystonia following magnetic resonance imaging in a patient with deep brain stimulation electrodes for the treatment of Parkinson disease. Case report. J Neurosurg 99:772-774.
3. Carmichael DW, Pinto S, Limousin-Dowsey P, et al. (2007) Functional MRI with active, fully implanted, deep brain stimulation systems: Safety and experimental confounds. NeuroImage 37:508-517.
4. Gorny K, Presti MF, Goerss SJ et al. (2013 ) Measurements of RF Heating during 3.0T MRI of a Pig Implanted with Deep Brain Stimulator. Magn Reson Imaging 31:783-788
5. Min HK, Ross EK, Lee KH, et al. (2014) Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul 7:603-607
6. Phillips MD, Baker KB, Lowe MJ, et al. (2006) Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus--initial experience. Radiology 239:209-216.
Figures