5431
Detailing radio frequency heating induced release of a fluorescent model drug attached to a thermoresponsive polymer carrier: A 7.0 T thermal MR study1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany, 2School of Chemical Engineering, University of New South Wales, Sydney, Australia, 3Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany, 4MRI.TOOLS GmbH, Berlin, Germany, 5Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz association, Berlin, Germany
Synopsis
Utilizing the RF pulses of the MR system for MR imaging, controlled RF heating and temperature monitoring with MRTh in an integrated system is appealing for MR guided thermal interventions. In this work, we demonstrate for the first time the applicability of our integrated system for targeted drug delivery using a pH- and thermoresponsive polymer. Upon RF heating to T>LCST, the polymers released 50% of its load in 30min, and 89% in 120min. Temperature monitoring with MRTh and external fiber optic temperature sensors were well correlated, with a deviation of 0.2-0.9°C.
Introduction
Thermoresponsive carriers that release their load upon temperature stimuli are intriguing smart drug delivery systems1-2. For an effective and safe thermal drug delivery, an accurate spatial guidance and temperature control is essential. Utilizing RF transmission for MRI, controlled RF heating and MR temperature monitoring in an integrated applicator3-4 is conceptually appealing for advancing the capabilities of thermal targeted delivery of drugs, MR sensitive probes or other loads. Here we demonstrate the applicability of an RF heating system integrated into a 7.0T whole body MR scanner for drug release using a pH- and thermoresponsive polymer carrier loaded with fluorescent model drug.Materials and methods
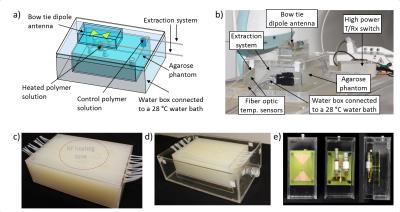
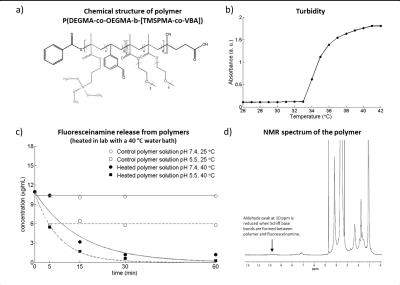
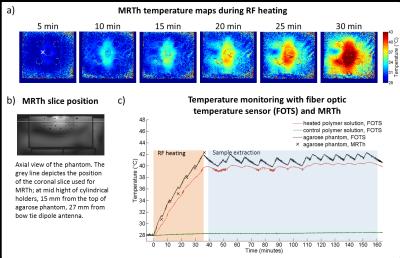
Based on electromagnetic field simulations5, an agarose phantom (dimensions=(18x28x9)cm³; σ=1.12±0.03S/m, ε=84.4±1.4) was constructed with seven sample holders and 15 PTFE tubes for fiber optic temperature sensors (FOTS) (Fig.1a-d). The phantom was positioned in a water bath (28°C) thermally regulated by a bath circulator6. An extraction system enables sample extraction during experiments without repositioning the patient table (Fig.1b). For RF heating, a bow tie dipole antenna3 (Fig.1e) was located above the central sample holder (Fig.1a) which was filled with a pH7.4 polymer7 solution. A control sample of the same solution was positioned outside of the RF heated area (Fig.1a). A custom build transmit/receive switch8 was used to support high peak power (MR imaging) and high average power (RF heating). Temperature maps were calculated based on the proton resonance frequency shift9 and a dual gradient-echo technique (FOV=(300×300)mm2, TR=61ms, TE1/TE2=6.34ms/11.44ms, spatial resolution=(1.5×1.5×4.0)mm3)10-11. Three FOTS12 were used as external references. The thermoresponsive polymer P(DEGMA-co-OEGMA-b-[TMSPMA-co-VBA])7 was used (Fig.2a). The lower critical solution temperature (LCST) was determined by turbidity measurents at 520nm13. Fluoresceinamine was loaded to the polymers as a drug model by formation of Schiff base bonds in the presence of triethylamine catalyst7. Prior to experiment in the MR scanner, the temperature induced release of fluoresceinamine was determined to be later used as reference. The polymer solutions, prepared at pH5.5 and pH7.4, were held at 25°C or heated at 40°C in a water bath for 60min. During this time small samples were extracted from the polymer solutions and passed through a size exclusion chromatography column14 where the released fluoresceinamine (347Da) is retained while the polymers (15kDa) with the attached fluoresceinamine were collected and analyzed in a fluorescence reader15. 1H-NMR spectroscopy was performed at 300MHz16. RF heating experiments were performed in a B0=7.0T MR system17. The heating paradigm consisted of 6x5min RF heating (Pavg=100W) interleaved with MR thermometry. After reaching 40°C in the heated polymer solution, the temperature was maintained for 120min with subsequent 2min RF heating periods. During this time, small samples of the polymer solutions were extracted, passed through size exclusion chromatography columns and analyzed in a fluorescence reader15.Results
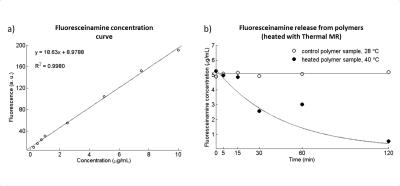
Turbidity measurement showed a LCST=34°C (Fig.2b). Water bath heating experiments showed little or no release of fluoresceinamine from polymer solutions held at T<LCST, except the initial 40% release from pH5.5 solution due to Schiff base bond breakage in an acidic environment (Fig.2c). Solutions at T>LCST showed almost complete release over 60min with a faster release for pH5.5 versus pH7.4 solutions. The aldehyde peak at 10ppm of 1H-NMR spectrum of the polymer (Fig.2d) is reduced when Schiff base bond is formed between the polymer and fluoresceinamine, which potentially allows the determination of drug release using 1H-MRS. Temperature in the heated polymer solution increased from 28°C to 40°C after 30min of RF heating, while the control sample maintained at 28°C (Fig.3). MRTh measurements were consistent with FOTS readings, with 0.2-0.9°C of deviation (Fig.3c). The release of fluoresceinamine from the polymers was verified in the heated solution. The fluoresceinamine concentration curve used to quantify these results has an R²=0.998 for linear fit indicating good fidelity (Fig.4a). After 30min at 40°C, 50% of loaded fluoresceinamine was detached from the polymers (Fig.4b). After 120min, the release was 89% (Fig.4b). The control solution did not show apparent fluoresceinamine release (Fig.4b).Conclusion
This work demonstrated the applicability of an RF heating system integrated in a 7.0T MR scanner for temperature induced release of a model drug attached to a thermoresponsive polymer. Our approach adds a thermal intervention dimension to an MR imaging device and provides an ideal testbed for the study of temperature induced release of drugs, MR probes and other agents from thermoresponsive polymers, nanogels and other carriers.Acknowledgements
This work was supported in part (Y.J., H.W., T.N., L.W.) by the German Federal Ministry of Education and Research, “KMU-innovativ”: Medizintechnik 13GW0102.
We would like to thank Brigitte Schlegel for acquiring the NMR spectrum.
References
1 McDaniel JR, et al., Int J Hyperthermia, vol. 29, pp. 501-10, 2013;
2 Abulateefeh SR, et al., Macromol Biosci, vol. 11, pp. 1722-34, 2011;
3 Winter L, et al., PLOS ONE 2013, 8(4):e61661;
4 Winter L, et al., Radiation Oncology, 2015;10(1):201;
5 Sim4Life, Zurich Med Tech, Zurich, Switzerland;
6 Dunn AE, et al., Polymer Chemistry, vol. 5, pp. 3311-3315, 2014;
7 Y Ji, et al., ISMRM Workshop on UHF MRI, 2016; 19;
8 Artic A10, ThermoScientific, MA, USA;
9 Ishihara Y, et al., Magn Reson Med 1995, 34(6):814-823;
10 Rieke V, J Magn Reson Imaging 2008, 27: 376–390;
11 Winter L, et al., Int J Hyperthermia, 2015, 32:63-75;
12 Omniflex, Neoptix, Quebec, Canada;
13 Safire2, Tecan, Zurich, Switzerland;
14 Bio-Spin, Bio-Rad Laboratories, Inc., CA, USA;
15 Gemini XPS, Molecular Devices, CA, USA;
16 AV 300, Bruker Biospin, Ettlingen, Germany.
17 Magnetom, Siemens Healthcare, Erlangen, Germany
Figures