5429
MR Thermometry-guided Ultrasound Hyperthermia of User-Defined Regions Using the ExAblate Prostate Ablation Array1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Radiation Oncology, University of California San Francisco
Synopsis
We have implemented a real-time MR thermometry-guided system for ultrasound hyperthermia delivery within user-defined regions and validated it in phantom experiments. It was based on the commercial ExAblate prostate transducer, RTHawk real-time MRI system and featured accelerated PRFS MR thermometry and automated steering of the ultrasound focus to ensure uniform heating of the entire region of treatment.
Objectives
Hyperthermia (HT, 40-45°C, 30-60 min) has been combined successfully with several cancer treatment modalities, such as radiation, chemotherapy and drug delivery to enhance treatment efficacy1,2. Clinical studies have demonstrated feasibility of safe application of prostate hyperthermia with endorectal ultrasound applicators3.
The use of a commercial focused ultrasound (FUS) system to achieve HT has been previously demonstrated4. However, precise delivery of uniform HT to organs such as prostate remains challenging, since it requires diffused energy depositions, sustained over long durations. To achieve the maximum therapeutic benefit, the region of treatment (ROT) should conform to the shape of the lesion.
The goal of this project was to implement a software control and monitoring system for MR thermometry-guided hyperthermia delivery using the ExAblate prostate array that would achieve uniform stable heating of a large region of treatment, while allowing the user to precisely control the shape of the ROT. Spatial control was achieved by real time multipoint temperature sampling, continuously steering of the ultrasound focus towards the coldest areas of the ROT while controlling the power output of the transducer.
Methods
System overview
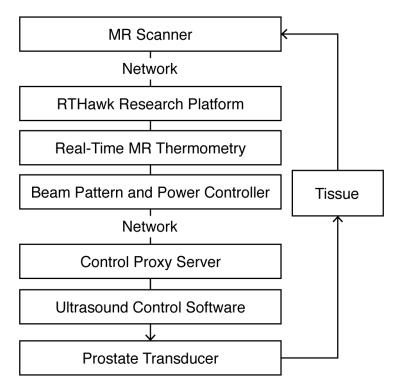
The hyperthermia delivery system was based on a 3T MR scanner (GE Healthcare, Waukesha, WI), ExAblate 2100 prostate array (InSightec, Haifa, Israel), and RTHawk real-time MRI system (HeartVista, Inc., Menlo Park, CA). Imaging was performed with the body coil.
MR Thermometry Sequence
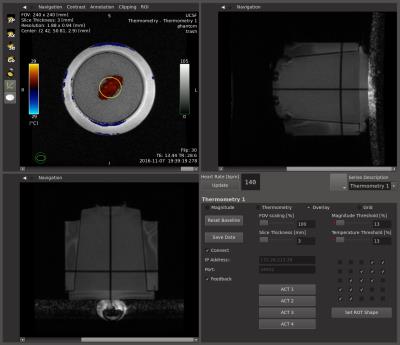
An accelerated real-time MR-HT application (fig. 1) was implemented on the RTHawk platform. It allowed continuous acquisition and reconstruction of temperature maps with minimal latency using an SPGR pulse sequence, a real-time PRFS thermometry reconstruction pipeline and a custom interface for data visualization and prescription.
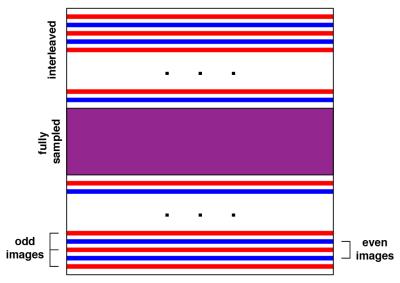
The pulse sequence implemented a shared k-space with keyhole acceleration technique5, where the central part of k-space was fully sampled and was acquired for every imaging frame. Peripheral lines of k-space were split into alternating sets that were updated less often (fig. 2).
Beam Pattern and Power Controller
The beam controller module was developed within the MR-HT application. Temperature values were sampled on a 5x5 grid that was placed over the region of treatment.
The FUS system was set up with a set of 25 sub-sonication patterns that matched the cells of the grid. The controller module determined the next focal point and power level, and communicated that over the network to the FUS system via a Control Proxy Server application (fig. 3).
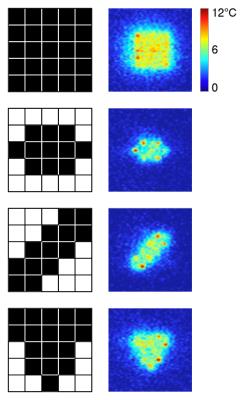
The system directed the beam towards the cell with the lowest measured temperature. After the entire ROT reached a temperature within 0.5° of the target, power was linearly decreased. To control the shape of the region, individual cells of the grid could be turned on and off in the application user interface.
Experimental Setup
The system was evaluated in experiments with a tissue-mimicking phantom for prolonged exposures with a target temperature increase of 7°C from baseline, maximum acoustic power output of 26 W at 2.3 MHz. Four shapes of the region of treatment were implemented: square, pill-shaped, diagonal, and triangular. MR thermometry (256x128 matrix, TE= 13 ms, TR = 29 ms, FOV = 24 cm) was acquired with 32 lines of central k-space at every frame and an interleave factor of 2 for peripheral k-space, resulting in a 1.6x acceleration over the fully-sampled acquisition.
Results and Discussion
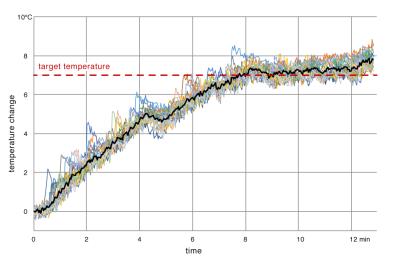
Our results showed patterns of heating that closely matched the prescribed regions (fig. 4). It took around 7 minutes for the whole region of treatment to reach the target temperature (fig. 5). After that, stable heating was maintained for another 5 minutes with the mean temperature in the region within 1°C of the target temperature of 7°C. The cells at the periphery of the ROT experienced higher fluctuations in temperature due to heat dissipation into the surrounding tissue, while the inner cells showed little variation.
In order to reach the target temperature within a large region in a reasonable time, the transducer initially operated at high power (26W) that was reduced after approaching the target temperature. Fast imaging was nevertheless important to minimize the risk of overheating the tissue at the beam focus during the intervals between focus changes. The shared k-space with keyhole acceleration technique allowed to improve the acquisition frame rate, achieving 2 s update time per frame and providing for a smoother heating.
In conclusion, we have implemented a real-time MR thermometry-guided system for hyperthermia delivery within constrained regions with the ExAblate prostate array and validated it in phantom experiments. Future work includes implementation of faster accelerated imaging, investigation of heating complex 3D regions, and in-vivo validation.
Acknowledgements
This work was supported in part by a Research Award from the Focused Ultrasound Foundation and research support from InSightec.References
1. Mallory M, Gogineni E, Jones GC, Greer L, Simone CB, 2nd. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit Rev Oncol Hematol 2016;97:56-64.
2. Hurwitz MD. Today's thermal therapy: not your father's hyperthermia: challenges and opportunities in application of hyperthermia for the 21st century cancer patient. Am J Clin Oncol 2010;33(1):96-100.
3. Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, Hynynen K, Kaplan ID. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber Cancer Institute study 94-153. Cancer 2011;117(3):510-516.
4. Salgaonkar VA, Prakash P, Rieke V, Ozhinsky E, Plata J, Kurhanewicz J, Hsu IC, Diederich CJ. Model-based feasibility assessment and evaluation of prostate hyperthermia with a commercial MR-guided endorectal HIFU ablation array. Med Phys 2014;41(3):033301.
5. Zaitsev M, Zilles K, Shah NJ. Shared k-space echo planar imaging with keyhole. Magn Reson Med 2001;45(1):109-117.
Figures