5428
In Vivo Targeting Accuracy Assessment of a MR-guided Focused Ultrasound Device (MRgFUS) Equipped with Tracking Coils1Bioengineering, University of Utah, Salt Lake City, UT, United States, 2Physics, University of Utah, Salt Lake City, UT, United States, 3Radiology, University of Utah, Salt Lake City, UT
Synopsis
MR-guided focused ultrasound (MRgFUS) ablation treatments can be improved with more accurate and faster treatment planning. In this study, a fast, automatic tracking coil system is employed to predict the ultrasound transducer focus for treatment planning purposes. The accuracy of the geometric focus predictions was assessed in vivo with a breast-specific MRgFUS device. This study reports a targeting accuracy of 3.1 ± 1.4mm, measured as the average Euclidean distance between the volumetric center of mass of thermal sonications and their predicted focal locations.
Purpose
The non-invasive nature of MRgFUS procedures is both a significant advantage of and technical challenge for the developing surgical technique. Advancements in MRI have allowed for precise anatomical targeting and monitoring of MRgFUS ablations. However, streamlining of treatment planning could reduce treatment times. Locating the geometric focus (GF) at the start of ablation is typically an iterative, time-consuming process of performing low temperature sonications and moving the MR thermometry slices in search of the sonication focal center.1-2 To improve the accuracy of MRgFUS ablations and reduce treatment planning time, a MR tracking coil system has been developed for a breast-specific MRgFUS device to automatically predict the transducer GF.3 Our study assesses the in vivo accuracy of this tracking coil system.Methods
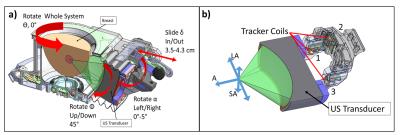
An intramuscular VX2 tumor model in White New Zealand rabbits (N=4) was used in this study. Tumors were injected via cell suspension (~1 x 106 cells in a 50:50 media/Matrigel solution) bilaterally into rabbit thigh muscles three weeks before ablation. Ablation was performed using a breast-specific MRgFUS system4 (Figure 1) inside a Siemens Trio 3T MRI scanner. Anesthetized rabbits were positioned on the MRgFUS device with the transducer positioned to allow acoustic access to the tumor (Φ = 45°, α = 0-5°, δ = 3.5 - 4.3 cm [Figure 1a]). A tracking coil system, previously developed by Svedin et al.3, was used to predict the GF of the transducer in MRI coordinates. Briefly, a one-dimensional readout sequence acquired the locations of three RF tracking coils (Figure 1b) that are used to calculate the predicted GF (computation time ~ 150 ms). After finalizing transducer position, tumor ablation was achieved by electronic steering of the ultrasound beam. Each animal received 4-8 individual sonications (~40 acoustic W, 30 seconds), monitored with PRF MR temperature imaging4 (MRTI). Oblique coronal 3D MRTI slabs were acquired parallel to the ultrasound beam path, such that each image dimension corresponded to an axis of electronic steering on the transducer (zero-filled interpolated to 1 mm isotropic [Figure 1b]). The volumetric center of mass (COM) of the individual sonications (n=28) was calculated after masking temperatures below 15% of the peak temperature. Electronically steered foci locations were defined in context of the local MRTI image coordinates relative to the GF in each rabbit. Targeting accuracy of the MRgFUS device was assessed by finding the Euclidean distance between the sonication’s COM and their corresponding predicted focus coordinates.Results
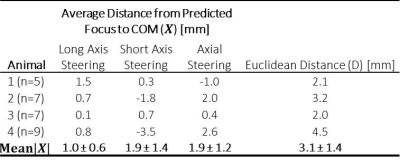
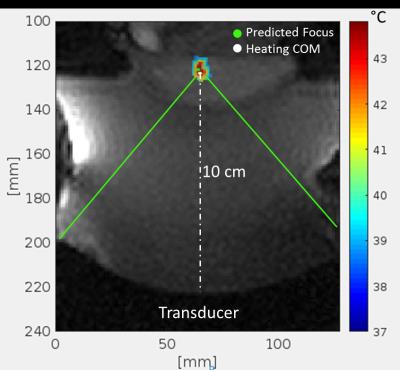
The results of the accuracy assessment for each animal are summarized in Table 1. The mean absolute error, independent of direction, between the predicted foci and measured COM ranged from 1.0-1.9mm for each steering direction, with an average Euclidean distance of 3.1 ± 1.4mm. An example sonication temperature profile in the rabbit muscle is shown in Figure 2, with a 1.9mm distance between the predicted steered focus location and calculated COM. Figure 3 shows representative temperature profiles, overlaid with predicted foci and COM values with varying amounts of spatial error.Discussion
The mean Euclidian distance between the transducer focus predicted by the MR tracking coils prior to ablation and the calculated COM was 3.1mm. The mean error is higher than previously measured in homogeneous phantoms with the same system (2.1 ± 1.1mm).3 However, this model was more technically difficult than the phantoms largely due to the ultrasound incidence angle and proximal position of the tumor to the skin when compared to a breast-shaped target. As a result, some ablations were focused <1.5mm from the skin, most likely leading to increased skin heating resulting in beam aberrations that can alter the focal location and profile shape. Despite the challenges of this in vivo ablation, the tracking coils were accurate and invaluable for predicting and planning the tumor ablations. As can be seen Figure 4, the predicted focus is within the central region of heating, suggesting that the intended target could still be treated, even when the COM is shifted from the predicted location. Finally, the COM calculation was influenced by near and far-field heating close to the focal location, potentially resulting in higher distance errors than there would be otherwise.Conclusion
The accuracy of RF tracking coils for planning FUS ablation treatments in a breast-specific MRgFUS device was found to be 3.1 ± 1.4 mm (n=28) during in vivo ablations of intra-muscular tumors in rabbits. The tracking coils in this study were validated as an alternative to time-consuming, iterative calibration sonications for finding the transducer focus during MRgFUS treatments. These results will accompany other studies in developing a standard for ablation targeting accuracy in the field of MRgFUS.Acknowledgements
This work was funded by NIH R01 CA172787.References
1. Ellis, S.. Rieke, V., Kohi, M., Westphalen A. Clinical applications for magnetic resonance guided high intensity focused ultrasound (MRgHIFU): Present and future. J. of Med. Img. and Rad. Onc. 2013;57(4):391-399.
2. Elias, J.W., Huss, D., Voss, T., et al. A pilot study of focused ultrasound thalamotomy for essential tremor. The New England Journal of Medicine. 2016;375(8):730-739.
3. Svedin, B., Beck, M., Hadley R., et al. Focal point determination in magnetic resonance-guided focused ultrasound using tracking coils. Mag. Res. In Med. 2016;7691):206-213.
4. Payne, A., Vyas, U., de Bever, J., et al. Design and characterization of a laterally mounted phased-array transducer breast-specific MRgHIFU device with integrated 11-channel receiver array. Med. Physics. 2012;39(3): 1552-1560.
Figures