5421
MR Relaxation Properties in MR Coagulation for Vascular Repair1Department of Physics, University of Massachusetts Lowell, Lowell, MA, United States, 2Martinos Center, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 3Robin Medical, Inc., Baltimore, MD, United States
Synopsis
As part of a project to develop MR-induced RF heating of a coagulable biomaterial for minimally invasive repair of vascular lesions, we investigate the relationship between the heat coagulation behavior and MR relaxation properties of egg white. This protein solution is a good model for the analogous behavior of human serum albumin solution, an optimal (but expensive) biomaterial for MR coagulation. We find that large changes in both T2 and the width of the magnetization transfer spectrum clearly indicate the temperature at which the coagulation process occurs. T2 and MT weighted MR images identify coagulated material in an aneurysm phantom.
Purpose
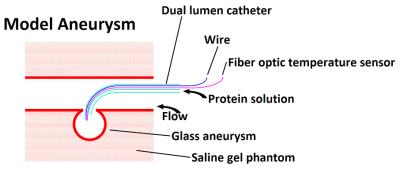
Magnetic Resonance Mediated Radiofrequency Coagulation (MR coagulation) employs the RF heating effect of the scanner to coagulate a biomaterial for repair of a vascular defect.1,2 Coagulation of a protein biomaterial by MR-induced RF heating is a novel means to effect repair of vascular defects such as aneurysms or arteriovenous malformations. These defects are conventionally repaired by surgical clipping or by filling/occluding with endovascularly delivered wire coils.3 Our novel method, MR Coagulation, is intended to achieve a comparable result by coagulating a thermosetting material (such as a protein solution) delivered endovascularly by catheter and coagulated by RF-induced heating of an intracatheter resonant wire antenna in the MRI scanner. The purpose of this study is to measure the MR relaxation properties of a protein solution undergoing coagulation to determine the most definitive MR methodology enabling the detection of coagulation. Human serum albumin (HSA) is the most abundant protein in the blood, and has been used surgically to seal blood vessels4 and to stem diffuse bleeding.5 In an image guided MR coagulation procedure it is necessary to determine when coagulation is complete using its MR characteristics. Egg white, essentially a 10 wt% protein solution with about half the protein being ovalbumin, can be used as an inexpensive substitute for HSA for investigating heat coagulation behavior and MR relaxation properties. In this study, the MR relaxation properties of egg white were studied as a function of temperature and degree of coagulation to determine which contrast parameters would be most appropriate for imaging studies. Additionally the coagulation of egg white by RF heating within a phantom aneurysm in an MRI scanner was performed, and images were obtained based on the relaxation parameters to test which parameter(s) would best ascertain the coagulation state in vivo. Aneurysm experiments were carried out using saline and water, and under conditions of flow and no flow.Methods
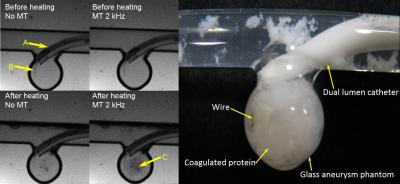
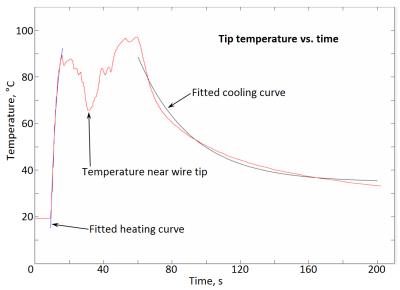
Experiments were performed on a Siemens 1.5 T (63.643 MHz) MRI scanner. A heating system was constructed to regulate the sample temperature inside the magnet. Egg white in 10 mm NMR tubes was brought to equilibrium at seven temperatures (20, 30, 40, 50, 60, 70 and 37 °C) in sequence. Separate runs with fresh sample were performed for each relaxation time, using inversion recovery, CPMG or magnetization transfer (MT) pulse sequences to measure the water spin-lattice relaxation time T1, spin-spin relaxation time T2, or full width at half maximum (FWHM) of the MT spectrum respectively. Also, relaxation parameters of raw egg white and egg white after coagulation at 70 °C were measured in the MRI scanner at room temperature (20 °C) to determine optimum inversion time, echo time and offset frequency for the pulse sequences. Finally coagulation of egg white within a glass aneurysm phantom by RF heating inside MRI scanner was carried out and images of the phantom aneurysm were taken to observe the state of coagulation.Results
It was found that water T2 and MT gave the most definitive indication of the change from uncoagulated at low temperature to fully coagulated at 60 °C, while water T1 showed only the expected gradual increase with temperature, and no response to coagulation.Discussion
In pure liquid water, T1 exhibits a minimum value when the correlation time for molecular reorientation matches the inverse of the Larmor frequency; as the correlation time either increases or decreases, T1 will increase. Unlike T1, T2 decreases as the correlation time increases.4 In the presence of a soluble or coagulated protein, chemical and spin exchange between bulk water protons, bound water protons and protein protons complicates the relaxation behavior. Because T2 is affected strongly by “static” frequency molecular motions, and spectral width of magnetization transfer is affected by the spin exchange between free water and bound protons, they are expected to be influenced strongly by the transition of solution proteins from the ordered to the agglomerated state, as borne out by our results. T2-weighted images are quick and easy to measure with low SAR, but the images showed artifacts due to the rapid dephasing caused by magnetic susceptibilities difference in different material.Conclusions
Relaxation experiments show that egg white protein fully coagulates at 60 ºC, which is relatively easy to achieve by MR-induced heating of an intracatheter antenna. MR coagulation has potential as a means to effect embolization of vascular defects. Means to produce a more consolidated coagulum and to reduce downstream flow of particles need to be developed. MT weighted imaging is expected to be the optimum method to establish the coagulation condition of the biomaterial.Acknowledgements
This research is supported by the Center for the Integration of Medicine and Innovative Technology (CIMIT) grant 13-1180/grant W81XWH-09-2-001 from U.S. Army Medical Research and Materiel Command (USAMRMC) and NIH grants RR023009 and P41RR14075.References
1. Cohen O, Ackerman JL. Repair of vascular defects using MR radio frequency coagulation. Joint Annual Meeting, International Society for Magnetic Resonance in Medicine and European Society for Magnetic Resonance in Medicine and Biology, Milan, Italy, May 10-16, 2014.
2. Zhao M, Ackerman JL. Water relaxation parameters and the state of protein coagulation for vascular repair by MR coagulation. Seventh Annual Image-Guided Therapy Workshop, Cambridge, MA, September 18-19, 2014.
3. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006;355:928-939.
4. Takao H, Murayama Y, Yuki I, et al. Endovascular treatment of experimental aneurysms using a combination of thermoreversible gelation polymer and protection devices: feasibility study. Neurosurgery 2009;65:601-609.
5. Mueller GR, Wolf RF, Hansen PD, Gregory KW, Prahl SA. J Gastrointest Surg 2010;14:1764-1769.
Figures