5417
In-Bore MRI-Guided and Monitored Laser Ablation for Renal Malignancy: Outcome Data from 46 Treated Tumors with 24-Month Median Follow-up1Radiology and Imaging Sciences, Emory University Hospitals and School of Medicine, Atlanta, GA, United States, 2Interventional MRI Program, Emory University Hospitals and School of Medicine, Atlanta, GA, United States, 3Radiology and Imaging Sciences, Interventional MRI Program, Emory University Hospitals, Atlanta, GA, United States, 4Pathology, Emory University Hospitals and School of Medicine, Atlanta, GA, United States, 5Urology, Emory University Hospitals and School of Medicine, Atlanta, GA, United States
Synopsis
Interventional MRI technology has been used to guide and monitor renal ablation procedures because of its ability to provide online feedback on ablation progress and determine treatment endpoints based of individual tumor responses. The field of Interventional MRI lacks the abundance of outcome data that provide the evidence for the added value of using MRI technology to guide interventions. We report the long-term efficacy data for interactive guidance and real-time monitoring of 46 renal ablation procedures performed entirely within an interventional MRI suite using a short introducing needle and a flexible laser fiber.
Introduction & Purpose:
Percutaneous ablative treatment has become a viable treatment option for selected patients with localized malignant renal neoplasms. The primary ablative technologies used are cryoablation and radiofrequency ablation (RFA), commonly performed under CT or ultrasound guidance. The use of MRI guidance has shown an added value for intraprocedural confirmation of a tumor-free ablation zone, thereby potentially reducing the incidence of residual /recurrent neoplasms 1, 2. MRI guidance of these procedures has, in our experience as in others’, been hampered by the cumbersome handling of cryoprobes and RFA probes and their cablings within the already limited room available within the MRI gantry, particularly when utilizing superconducting magnet designs. The aims of this investigation are to a) describe patient tolerance and complication rates associated with in-bore MRI-guided and monitored laser ablation of renal malignancies; and b) report the long term local control data over a median follow-up duration of 24 months.Patients & Methods:
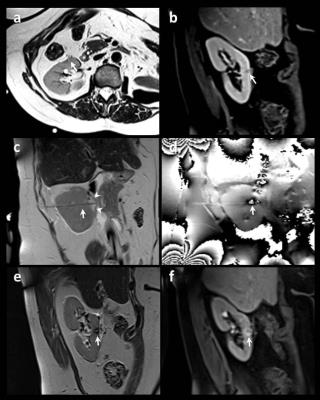
33 patients (17M, 16F, age=29-88y) with 50 renal masses underwent biopsies (48 MRI-guided & 2 CT-guided) followed by in-bore MRI-guided laser ablations. Procedures were performed within an interventional MRI suite equipped with 1.5T wide bore scanner. Interventions were performed under general anesthesia, entirely within the scanner bore while viewing real-time image updates on an in-room monitor. Interactive visualization on a tri-orthogonal plane True-FISP sequence (TR/TE/FA=2700/84/170°) was used to guide a 14.5-cm-long, 14G MRI-compatible introducing needle into the targeted lesion. 20G FNA and/or 18G core samples were obtained. A laser fiber with 15mm diffusing tip encased in 5.5 F cooling catheter (Visualase, Texas, USA) was then introduced into the target lesion through the pre-existing short 14G introducing needle. The optic fiber and cooling tubing were extended through a waveguide to a 30w laser generator located outside the MRI room. A test dose of diode laser energy (980nm,30sec,9W) was applied to verify the location of ablation nidus on real-time temperature and cumulative damage estimate mapping(TE/TE=24/10). Subsequently, ablative energy dose was delivered (27W for cycles of 90-240 sec) with treatment endpoint based on on-line thermal monitoring of growing ablation. Fiber repositioning for additional ablation was conducted as needed. Final ablations were evaluated on a repeat set of pre-ablation scans consisting of TSE-T2 and pre- and post-contrast VIBE scans (Fig. 1).Results:
Four biopsies returned benign pathology. This analysis therefore includes 46 laser ablations of malignant renal tumors. Biopsy results showed one renal metastasis from lung cancer and 45 RCCs (22 clear cell, 11 papillary, 2 chromophobe, 7 oncocytic, 1 poorly differentiated high grade, 2 not specified). Target tumor sizes were 0.7-4.5 cm (29 right-, 17left-sided, 17 upper pole, 10 lower pole, 19 interpolar). 10 patients (30%) had a single kidney, 6 patients (18%) had prior ipsilateral partial nephrectomy, and 2 lesions were recurrent masses at prior cryoablation margins. Access to the desired part of the kidney using the 14.5-cm-long introducing needle was feasible in all cases, including one morbidly obese patient, with no space constraints encountered within the 70-cm magnet bore. The flexible nature of optic laser fibers eliminated the complexity of handling bulky ablation probes, and the traction exerted by their cablings and fitted the MRI environment. The short ablation cycle facilitated accurate temperature mapping during controlled suspended ventilation without the need to implement motion correction algorithms. The median applied laser energy was 22694J per lesion, with dosage calibrated based on real time feedback of tumor response to ablation. Nine small or moderate self-limited perinephric hematomas occurred as well as one delayed ablation bed abscess. Otherwise, no early or delayed complications were encountered. Follow-up durations ranged between one week and 56 months (median follow-up = 24 months). No residual or recurrent neoplasm was identified in any patient throughout these extended follow-up durations.Discussion & Conclusion:
Interactively guided and monitored renal ablation procedures performed entirely within an interventional MRI suite are safe and well-tolerated by patients. Long-term efficacy data indicate a reliable local tumor control with 0% recurrence rate observed over extended follow-up durations (median = 24 months, maximum = 56 months). This efficacy is likely related to the improved visualization of tumor margins during guidance along with the temperature sensitivity of MRI that facilitates the performance of refined ablation procedures, tailored to tumor response rather than following standard pre-determined ablation parameters. The use of optical fibers within the MRI environment eliminates much of the complexity associated with handling of cryo- and RFA probes and has become an important factor in streamlining the workflow of a routine tumor ablation service in the MRI suite at our institution.Acknowledgements
No acknowledgement found.References
[1] Lewin JS, Nour SG, Connell CF, et al. Radiology. 2004; 232(3):835-45.
[2] Silverman SG, Tuncali K, vanSonnenberg E, et al. Radiology. 2005; 236(2):716-24.
Figures