5416
Mapping TMS immediate effects by concurrent TMS/fMRI using a dedicated high-sensitivity coil array1Division MR Physics, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Wien, Austria
Synopsis
In this work we show the feasibility of a novel concurrent TMS/fMRI setup based on two multi-channel receive arrays that allows for whole brain fMRI data acquisition during brain stimulation. We show that stimulation at frequencies of 1 and 10Hz, respectively, leads to distinct changes in BOLD signal. We also report increased TMS-related network effects with higher stimulation. This validity check sets the frame for efficacy studies investigating the TMS stimulation of cortical areas for modulating complex brain networks.
Introduction
Even though transcranial magnetic stimulation (TMS) has been studied for almost a century and is an FDA-approved clinical intervention, the effects of superficial cortical stimulation using TMS on local and whole-brain physiology are still poorly understood. We made first steps towards gaining insights into direct cortical effects of TMS by developing a dedicated multi-channel receive (RF) array for concurrent TMS/fMRI1. Consisting of an ultra-slim 7-channel RF-coil and an MR-compatible TMS-coil mounted on top of it, this setup allowed for high-resolution image acquisition and unimpeded simultaneous TMS stimulation in the human primary motor cortex2. Compared to a conventional setup using the TMS coil inside a birdcage coil, our setup improves SNR up to a factor of 5 at target depth (3cm) and allows for parallel imaging, enabling more detailed and faster image acquisition, as previously demonstrated in a stimulation study in the motor cortex2.
However, using one RF-coil array restricts data acquisition to parts of the brain close to the RF-coil. Consequently, mapping of stimulation effects is limited to areas close to the stimulation site. In order to answer more complex research questions, such as TMS-related network modulation, we targeted the left DLPFC in the current study and used a second 7-channel RF-coil on the opposite of the stimulation site. Using this novel setup in combination with advanced fast and precise imaging and neuronavigation methods, we demonstrate acute local and network effects of TMS over the left DLPFC.
Methods
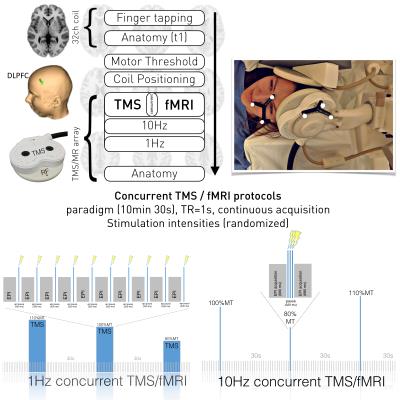
The study was performed on a 3T TIM Trio scanner (Siemens, Erlangen, Germany). Our TMS system included a MagProX100 stimulator and MRi-B91 MR-compatible TMS coil (Magventure, Farum, Denmark). In order to perform spatially exact stimulation, we employed neuronavigation and an additional spatial markers (visible in localizer scans) installed in the RF-coil allowing for backtracing of coil positioning throughout the whole data acquisition procedure.
Five right-handed female subjects (age: 24.9 ± 3.2 years) participated in the experiment. Anatomical images were acquired using a MPRAGE sequence (TR / TE / TI = 2300 ms / 4.21 ms / 900 ms, flip angle 9°, 160 slices, 1.25 mm slice thickness). Functional images were acquired using EPI sequence with TR/TE=1000/33ms, 28 slices, 1.5x1.5x3mm³. Experimental setup is displayed in Figure 1. FMRI data analyses were performed using SPM12. The design matrix comprised four regressors representing different stimulation amplitudes of 10Hz rTMS over left DLPFC.
Results
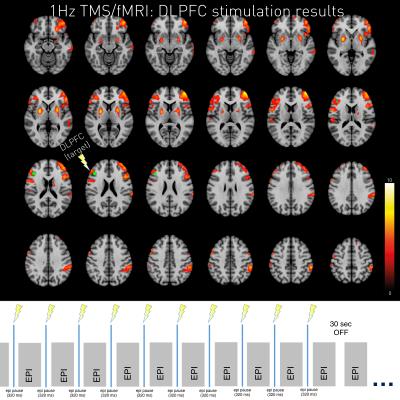
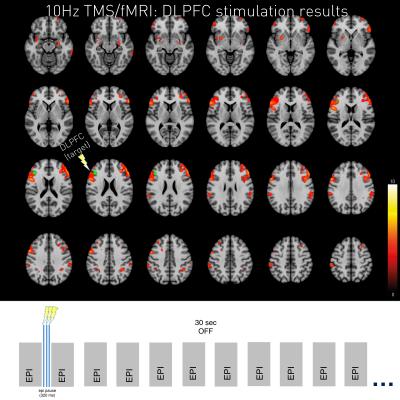
The new setup allowed for high-resolution whole-brain coverage of acute TMS over DLPFC effects as sensitive as to make effects visible on single-subject level. Both 1Hz stimulation and 10Hz stimulation led to BOLD changes at the stimulation site (left DLPFC) and in other areas of the DLPFC–network.
Interestingly, 10Hz and 1Hz stimulation evokes locally overlapping neural responses as well as frequency dependent activity (e.g. NAcc activity during 1Hz stimulation; compare Figure 2 & 3).
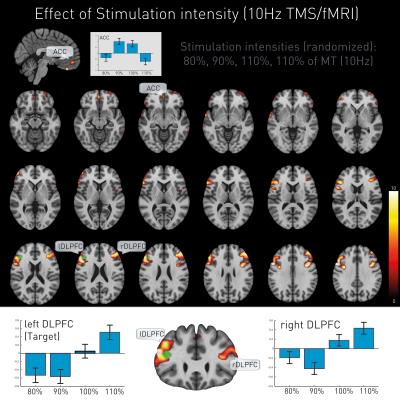
Additionally, TMS led to intensity-dependent local and remote activation changes. The stimulated target (left DLPFC) and its contralateral homologue (right DLPFC) showed proportional direct intensity related changes, while ACC manifested a more complex response pattern (Figure 4).
Discussion
Here we extend a previously reported single-array TMS/fMRI setup1 to whole-brain coverage by using two ultra-slim 7-channel RF-coils for enabling concurrent TMS/fMRI with high spatial resolution. Due to whole-brain image acquisition during TMS stimulation of the DLPFC we are able to capture not only the acute effects of TMS over the left DLPFC but also network-wide effects of the stimulation paradigm on an individual level.
We further show a dose-dependent neural response not only underneath the stimulation site but also on the contralateral DLPFC and apparent mediating responses in the ACC.
Conclusion
The feasibility of testing hypotheses on causal network interactions as shown by our TMS/fMRI setup allows understanding brain stimulation at the individual subject's level and provides the basis for precision medicine in neuropsychiatric disorders.Acknowledgements
We thank Tina Henneken and Anna-Lisa Schuler for technical assistance and comments.References
1 Navarro de Lara L, Windischberger C, Kuehne A, et al. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn Reson Med. 2015;74(5):1492-1501.
2 Navarro di Lara L, Tik M, Woletz M, et al. Boosting the sensitivity of concurrent TMS/fMRI in motor cortex using a dedicated MR coil array. Honolulu, USA: 21th Meeting of the Organization for Human Brain Mapping; 2015.
Figures