5409
Altered structure and function reflect chronic pain in patients with idiopathic trigeminal neuralgia1Department of Medical Imaging, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, People's Republic of China, 2Department of Neural and Pain Sciences, School of Dentistry, University of Maryland Baltimore, Baltimore, MD, United States, 3Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, Stomatological Hospital, Xi’an Jiaotong University Health Science Center, Xi'an, People's Republic of China, 4Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
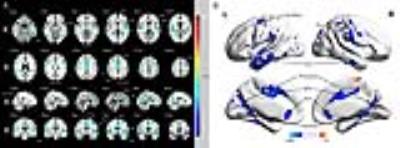
To testify the hypothesis of morphometric and functional alterations of patients with idiopathic trigeminal neuralgia (ITN), we displayed gray matter volume (GMV) reductions in the anterior and middle cingulate cortex (ACC and MCC), insula, and several regions of temporal lobe. Additionally, enhanced functional connectivity was revealed between right insula and ACC, medial prefrontal cortex, posterior cingulate cortex (PCC), and dorsal lateral prefrontal cortex in ITN patients. Furthermore, GMV of left inferior temporal gyrus negatively correlated with pain intensity and disease duration in patients, and connectivity of right insula-ACC was negatively associated with pain scores, depression, and anxiety ratings, respectively.
Introduction
Idiopathic trigeminal neuralgia (ITN) is a intense paroxysmal pain disorder characterized by unilateral lancinating attacks in the branches of trigeminal nerve. The etiology of ITN remains unclear; although researches suggest that most of ITN is due to microvascular compression of the trigeminal root by aberrantly formed blood vessels, called nerve vessel conflict (NVC) [1]. However, NVC is not exclusive to ITN patients, as an estimated 40% of healthy controls exhibit various degree of NVC [2], suggesting that other central mechanisms also play a role in the development of ITN. Task-related functional MRI study has reflected a state of maintained sensitization of the trigeminal nociceptive systems in ITN [3], but resting state functional connectivity in patients with ITN has rarely been studied before. Meanwhile, structural deficits including grey matter and white matter abnormalities may be accompanied by the changes of functional network. So we combined the morphometric and functional imaging features of ITN and correlate these with clinical traits, including pain intensity and emotional indexes.
Materials and methods
Thirty-eight ITN patients and 38 age and sex matched healthy controls were recruited according to the International Classification of Headache Disorders (ICDH-III). All participants rated the extent of their pain intensity using a visual analogue scale (VAS), and the Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Rating Scale (HAMA) were adopted to evaluate emotional deficits. Imaging data were collected on a 3.0-T GE scanner. High-resolution structural image was acquired using three-dimensional (3D) MRI sequence. Then the resting state fMRI (rsfMRI) data and diffusion tensor image (DTI) were obtained using echo planar imaging. Analyses of voxel based morphometry (VBM) and rsfMRI were both performed using Statistical Parametrical Mapping 8 (SPM8) in Matlab 2012a. Grey matter volume (GMV) was analyzed between ITN patients and controls using standard pipeline. Functional connectivity was performed using the seed correlation approach [4]. Seeds were centered on the peak voxels for the GMV clusters showing significant differences between the two groups. Group difference on voxel-wise GMV and functional connectivity were both performed with a voxel-wise threshold of uncorrected p< 0.005 and then corrected for multiple comparisons at the cluster level. Finally, DTI data processing and analysis were carried out using FMRIB Software Library (FSL) software. Tract-Based Spatial Statistics (TBSS) analyses were performed to examine the differences of fractional anisotropy (FA) and Mean diffusivity (MD) between ITN patients and healthy controls. The results were corrected using threshold-free cluster enhancement (TFCE) correction for multiple comparisons (p< 0.05, corrected). To test whether the results from group comparisons for GMV, DTI, and rsfMRI for ITN patients were related to clinical variables, we extracted data from the multi-modality MRI clusters and ran correlation analyses with disease duration, VAS scores, HAMA and HAMD scores by using SPSS software.Results
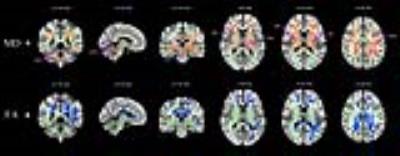
For VBM analyses, ITN patients displayed GMV reductions in regions including the anterior and middle cingulate cortex (ACC and MCC), insula, primary and secondary somatosensory cortex (S1 and S2), primary motor cortex (MI), premotor area (PMA), and several regions of temporal lobe. For DTI analysis, patients compared to controls had increased MD and decreased FA in the corpus callosum and the bilateral corona radiata, and increased MD with no FA changes across the bilateral superior longitudinal fasciculus (SLF), the internal and external capsule, the thalamus and brainstem. Additionally, enhanced functional connectivity was revealed between the right insula/S2 and ACC, medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and bilateral dorsal lateral prefrontal cortex (DLPFC) in ITN patients. Furthermore, GMV of left inferior temporal gyrus (ITG) negatively correlated with current pain intensity and disease duration in patients, and connectivity of the right insula/S2-ACC was negatively associated with pain intensity, depression, and anxiety ratings, respectively.Conclusion
We show distinctive structural and functional changes in ITN patients compared to controls. The grey matter, white matter and functional connectivity of ITN patients had multi-dimensional abnormalities across the whole brain. Moreover, GMV reduction of left ITG may become an alternative index of estimation for orofacial pain perception and disease duration, and negative correlations of right insula/S2-ACC connectivity to the pain severity and affective dysfunctions could be a useful marker for predicting the perceptive and emotional progression of the disease. These findings may help us to better understand the pathophysiology of the featured orofacial pain and provide an insight to facilitate the development of new therapies for ITN.Acknowledgements
We thank Dr. Massieh Moayedi for assistance with DTI analysis. This work was supported by the National Natural Science Foundation of China (No. 81301207).References
[1] Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. British Medical Journal. 2007, 334(7586):201-205.
[2] Kakizawa Y, Seguchi T, Kodama K, et al. Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg. 2008,108(3):483-490.
[3] Moissetl X, Villainl N, Ducreuxl D, et al. Functional brain imaging of trigeminal neuralgia. European Journal of Pain. 2011,15(2):124-131.
[4] Khan SA, Keaser ML, Meiller TF, et al. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain. 2014,155(8):1472-1480.
Figures