5405
Multimodal meta-analysis of neural correlates in first-episode drug-naïve major depressive disorder1West China Hospital of Sichuan University, Chengdu, People's Republic of China, 2West China Hospital of Sichuan University, People's Republic of China
Synopsis
Evidence of structural and resting-state functional brain abnormalities in MDD has been inconsistent. We conducted the first multimodal meta-analysis of voxel-based morphometry (VBM) and amplitude of low-frequency fluctuation (ALFF) studies in first-episode drug-naïve MDD patients. 15 VBM data setsand 11 ALFF data sets were included. A multimodal meta-analysis was used to highlight brain regions with both structural and functional abnormalities. The multimodal meta-analysis identified conjoint structural and functional differences in the left lateral orbitofrontal cortex and right supplementary motor area. Dissociated anatomical and functional brain abnormalities in MDD were also observed. Meta-analysis revealed in MDD a complex pattern of conjoint and dissociated structural and functional brain abnormalities in brain regions involved in cognition and emotional processing.
Purpose
Structural and resting state functional brain abnormalities have long been suspected in major depressive disorder (MDD) (1, 2) but the available evidence has been inconsistent, which is at least partly explained by considerable variation in sample size (too small a sample can yield both false-positive and false-negative findings), the patients’ demographic and clinical characteristics, and the imaging protocols used. Moreover, we know little about how structural and functional changes co-occur in MDD. To address this lack and minimize the effects of repeated episodes and antidepressant treatment, we conducted the first multimodal meta-analysis of voxel-based morphometry (VBM) (3) and amplitude of low-frequency fluctuation (ALFF) (4) studies in first-episode (FE) drug-naive MDD patients, using Seed-based d Mapping (SDM) (5).Method
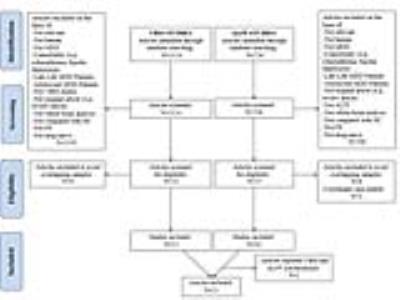
Meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA). A systematic search was conducted for VBM and ALFF studies in FE drug-naive MDD. Eventually, subjects included 471 FE drug-naive MDD matched with 521 healthy controls in VBM studies and 261 FE drug-naive MDD subjects matched with 278 healthy controls in ALFF studies ages 18-60. A voxel-wise meta-analysis using the SDM method was conducted on VBM studies on FE drug-naive MDD. Similarly, an SDM meta-analysis was conducted on ALFF studies. A subsequent multimodal meta-analysis (6) was used to highlight brain regions with both structural and functional abnormalities.Result
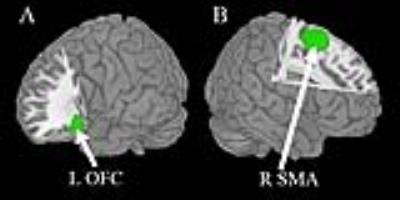
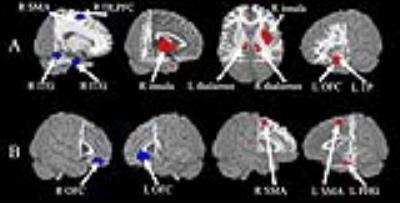
The multimodal meta-analysis identified conjoint structural and functional differences in the left lateral orbitofrontal cortex (lOFC) (i.e. increased gray matter and decreased brain activity) and right supplementary motor area (SMA) (i.e. decreased gray matter and increased brain activity) in MDD (Fig.2). Dissociated anatomical and functional brain abnormalities in MDD were also observed, characterized by reduced gray matter mainly localizing in the right dorsolateral prefrontal cortex (DLPFC), right inferior temporal gyrus (ITG) and increased gray matter in right insula extending to putamen, left temporal pole (TP), bilateral thalamus (Fig.3A), along with enhanced brain activity in the left parahippocampal gyrus (PHG) extending to hippocampus, left SMA and attenuated brain activity right lOFC (Fig.3B).Discussion
This is to our knowledge the first multimodal neuroimaging meta-analysis which attempts to localize the neural substrates of MDD by combining information from whole-brain VBM studies investigating GM with ALFF studies of spontaneous brain activity. The meta-analysis on FE MDD patients helps to distinguish the intrinsic brain features of the disease from potential effects of episode times, and the meta-analysis on drug-naïve MDD patients minimizes the interfere from medication effects. We found the increased GM in right insula, bilateral thalamus and left temporal lobe may putatively represent a specific character of early-stage MDD without long-term duration. Even so, the changes in above mentioned structure still supported the well-known ganglia-thalamocortical circuits (7), representing emotional, cognitive and somatic abnormalities respectively, involved in the physiological of MDD. FE drug-naive MDD patients showed increase in gray matter as well as decrease in brain activity in the left lOFC, and decrease in gray matter as well as increase in brain activity in right SMA, which could therefore serve as a specific region of interest template for future studies.Conclusion
The present meta-analysis revealed a complex pattern of neural abnormalities in drug-naive MDD patients, characterized by conjoint and dissociated structural and functional brain abnormalities in brain regions involved in cognition, emotional processing. These findings may contribute to a better understanding of the underlying pathophysiology and a better characterization of the abnormal structural and functional neural correlates of FE drug-naive MDD. Results from our multimodal meta-analysis demonstrate a close relationship between structural and functional brain abnormalities in MDD patients.Acknowledgements
This study was supported by the National Natural Science Foundation (Grant No.81030027) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1272) of China.References
1. Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in cognitive sciences. 2012;16:61-71.
2. Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biological psychiatry. 2015;77:223-235.
3. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805-821.
4. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & development. 2007;29:83-91.
5. Radua J, Rubia K, Canales-Rodriguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in psychiatry. 2014;5:13.
6. Radua J, Romeo M, Mataix-Cols D, Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Current medicinal chemistry. 2013;20:462-466.
7. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357-381.
Figures