5400
Comparison of fMRI and MEG language localization tasks—a prospective study of noninvasive presurgical functional mappingLi Zheng1, Jingwei Sheng1, Tianyi Qian2, Thomas Beck3, and Jia-hong Gao1
1Center for MRI Research, Peking University, Beijing, People's Republic of China, 2Siemens Healthcare, MR Collaborations NE Asia, Beijing, People's Republic of China, 3Siemens Healthcare, Application Development, Erlangen, Germany
Synopsis
In patients with intractable epilepsy, anomalous cortical organization could be observed and pre-surgical planning involving the localization of cortical language areas is critical. As a prospective study, we compared language localization using functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) with advanced source estimate method in 18 volunteers. Group analysis results showed something similar or discordant between fMRI and MEG activation. It is consider that fMRI technique combined MEG can used for the preoperative localization and postoperative functional evaluation in the future.
Purpose
In recent years, debate has grown about whether or not functional magnetic resonance imaging (fMRI) or magnetoencephalography (MEG) are suitable to be used as a noninvasive presurgical functional mapping tool in patients with brain tumors and/or epilepsies [1]. At the same time, there is a clinical motivation to combine fMRI data with an additional, noninvasive measurement that is more directly related to the underlying neural activity, such as MEG. The aim of this prospective study was to investigate the similarities and differences between language functional mapping using fMRI and MEG in healthy volunteers. This work may also improve the understanding of the neuron activity detected by fMRI, and the mechanism underlying the blood-oxygen-level-dependent (BOLD) signals.Method
A total of 18 healthy volunteers were recruited in this study. MRI datasets were acquired on a MAGNETOM Prisma 3T MR scanner (Siemens, Erlangen, Germany) with a 20-channel phased-array head-neck coil. Functional MRI data were obtained via a prototype simultaneous multi-slice EPI sequence (TR=2000ms, TE=30ms, number of slices=64, spatial resolution= 2mm x 2mm x 2mm), and the whole-head T1-weighted MR images (matrix size=256x224, slice thickness=1 mm) were acquired using the MPRAGE sequence (TR/TE=2530ms/2.98ms). MEG data were collected using the VectorViewTM whole-head MEG system (Elekta-Neuromag, Helsinki, Finland) with 306 MEG channels.
The task was to silently name actions and objects presented in simple line drawings. The experimental designs for the fMRI and MEG experiments were similar. For the MRI experiment, volunteers were asked to name objects based on schematic depictions, as shown in figure 1. For the MEG experiment, volunteers were asked to perform a similar task, modified to include pictures of objects with dissolved random lines; and the process included inter-stimulus intervals of 2 seconds in a random order and without repeats. The volunteers were then instructed to silently name each object as soon as the object was displayed on screen. The fMRI data were analyzed using a general linear model with random-effects in SPM12 software (http: //www.fil.ion.ucl. ac.uk/spm), while MEG source estimations were performed using the Fast-VESTAL source imaging method [2].
Results
Comparisons of the activation results obtained via fMRI and MEG are shown in figure 2. Activation results of fMRI are mainly distributed in the inferior frontal gyrus, insular cortex, and occipital cortex. By contrast, activation results of MEG are mainly distributed in the inferior frontal gyrus, postcentral gyrus, and temporal gyrus. In addition, strong visual activation appears in the prefrontal cortex on the map created by fMRI, but not on the map created by MEG. The results of group-analysis-based fMRI functional mapping of Broca’s and Wernicke’s area in 18 volunteers during an object-naming task are shown in figure 3. The results of MEG responses evoked by an object-naming task during 200-1000 ms poststimulus interval versus prestimulus baseline in 18 subjects are shown in figure 4. Activation of Broca’s area appears in both fMRI and MEG results, but activation of Wernicke’s area is stronger in the activation map of MEG.Discussion
The different results achieved via the two techniques occurred because the MEG control task was more effective. One possible explanation for the discrepancy in Wernicke’s area activation is that receptive language (measured via standard MEG tasks) and expressive language (measured via fMRI tasks) may dissociate during interhemispheric reorganization in individuals.
The determination of cortical areas involved in language function is an important aspect of presurgical planning. Patients with epilepsy demonstrate the highest incidence of anomalous language organization [3]. The FMRI technique detects haemodynamic changes associated with task processing in specific cortical areas, which is an effective method for lateralizing and localizing language areas accurately and noninvasively. However, the primary shortcoming of this method is that, despite good spatial resolution of sub-millimeters, the temporal resolution for fMRI is poor compared to that achieved by MEG. The MEG technique enables observation of the extracranial magnetic fields associated with performance of a task and tracks responses with millisecond precision. Therefore, both of these techniques should be compared in function localization, jointly inspected and verified. Finally they aid each other to provide better clinical results.
Conclusion
The results of our fMRI experiment are consistent with previous similar fMRI studies [4, 5]. In addition, we obtained analysis-based fMRI localization of the Broca’s area and Wernicke’s area. The results of MEG showed some accordance with fMRI results in terms of the localization in Broca’s area. MEG showed more precise localization, however, in Wernicke’s area. FMRI and MEG may tap into different aspects of language that dissociate in the event of cortical reorganization. The discordant results may provide valuable additional information. The fMRI technique combined with MEG offers the potential for better preoperative localization and postoperative assessment in the future.Acknowledgements
NoneReferences
[1] Huang C W, Huang M X, Ji Z, et al. High-resolution MEG source imaging approach to accurately localize Broca’s area in patients with brain tumor or epilepsy [J]. Clinical Neurophysiology, 2016, 127(5): 2308-2316.
[2] Huang M X, Huang C W, Robb A, et al. MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images [J]. NeuroImage, 2014, 84: 585-604.
[3] Risse G L, Gates J R, Fangman M C. A Reconsideration of Bilateral Language Representation Based on the Intracarotid Amobarbital Procedure [J]. Brain & Cognition, 1997, 33(1):118-32.
[4] Pang E W, Wang F, Malone M, et al. Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI [J]. Neuroscience letters, 2011, 490(3): 215-219.
[5] Liljeström M, Hultén A, Parkkonen L, et al. Comparing MEG and fMRI views to naming actions and objects [J]. Human brain mapping, 2009, 30(6): 1845-1856.
Figures
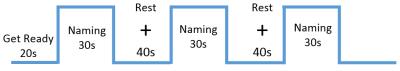
Figure 1. Schematic depiction of object naming task.
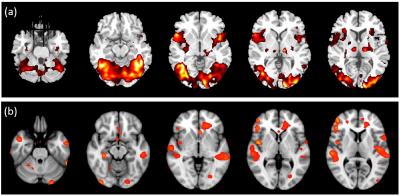
Figure 2. Comparison of (a) fMRI and (b) MEG based Fast-Vestal activation results.
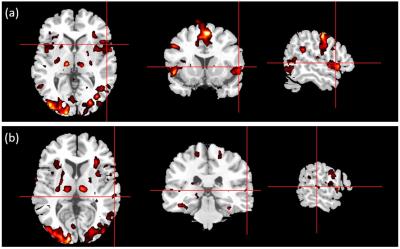
Figure 3. Group-analysis-based fMRI localization of (a) Broca’s area and (b) Wernicke’s area in ten volunteers during object-naming task.
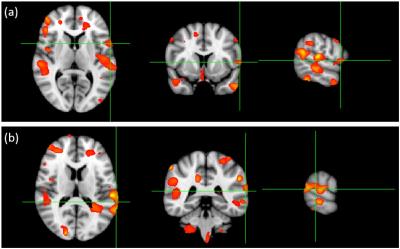
Figure 4. Group analysis-based MEG localization of (a) Broca’s area and (b) Wernicke’s area in ten volunteers during object-naming task.