5396
Neurochemical Basis of the BOLD Change in Attention Control - a combined task-based fMRI and 1H-MRS1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Special Education and Counselling, The Education University of Hong Kong, Hong Kong, Hong Kong, 4Sau Po Centre on Ageing, The University of Hong Kong, Hong Kong, Hong Kong, 5Department of Social Work and Administration, The University of Hong Kong, Hong Kong, Hong Kong, 6Philips Healthcare, Hong Kong, Hong Kong, Hong Kong, 7Department of Management Sciences, City University of Hong Kong, Hong Kong, Hong Kong, 8Department of Medicine, Queen Mary Hospital, Hong Kong, Hong Kong, 9Alzheimer's Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
fMRI can indirectly measure brain activity, but the biochemical underpinnings of the BOLD changes are still unknown. Nevertheless, 1H-MRS can bridge such gap by measuring Glx [summation of glutamate (Glu) and glutamine (Gln)], where Glu is one of the mediators of neurovascular coupling. In this study, we aim to elucidate the complex relationship between attention control (numerical Stroop) and its associated neurochemical changes by combining the biochemical information from task-based fMRI and 1H-MRS. Our result showed that the anterior cingulate cortex was positively correlated with Glx. This is the first study providing neurochemical explanation of the BOLD change during attention control task.
Purpose
Functional magnetic resonance imaging (fMRI) can indirectly measure brain activity because its blood oxygen level-dependent (BOLD) signal largely depends on neurovascular coupling, which refers to the processes by which neural activity influences the haemodynamic properties of the surrounding vasculature. Nevertheless, the biochemical underpinnings of such BOLD changes cannot be elucidated using fMRI alone. A promising technique for bridging the gap, by virtue of measuring in-vivo brain metabolite concentrations, is proton magnetic resonance spectroscopy (1H-MRS). One of the important metabolites which 1H-MRS can detect is Glx [summation of glutamate (Glu) and glutamine (Gln)], where Glu is one of the mediators of neurovascular coupling and its concentration would influence the BOLD signal changes.1 In this study, we aim to elucidate the complex relationship between attention control (numerical Stroop) and its associated neurochemical changes by combining the biochemical information obtained from task-based fMRI and 1H-MRS.Methods
A total of 90 healthy subjects will be recruited for the entire research project, approximately 15 subjects in each decade between 20 and 90. Potential subjects will be interviewed to gather socio-demographic data and undergo neuropsychological assessments. Only subjects with scores ≥28 in MMSE and ≥26 in MoCA will be included in the study. Written informed consent will also be obtained. 37 subjects were currently scanned. All experiments were performed with 3.0T Philips scanner using a standard head coil. Structural images were acquired with 3D fast field echo sequence (3D-T1-FFE sagittal). For the 1H-MRS part, PRESS (TR/TE = 2000/39 ms) single voxels of 2 x 2 x 2 cm3 were placed in the ACC (Figure 1a). Choline (Cho), creatine (Cr), N-acetyl aspartate (NAA), myo-inositol (mI), and summation of glutamate and glutamine complex (Glx), were measured and quantified using internal water as reference by QUEST in jMRUI (4.0)(Figure 1b,c). Cerebrospinal fluid (CSF) normalization, water content correction for grey matter, white matter and CSF, and correction factors for T1 and T2 relaxation were also implemented. The task-based functional images were collected by using a gradient-echo-planar sequence sensitive to blood-oxygen-level-dependent (BOLD) contrast in a numerical Stroop task (Figure 2). Data analysis was performed using Statistical Parametric Mapping (SPM12). The preprocessing process includes motion correction, normalization, and smooth with a Gaussian kernel of 8 mm FWHM (full width at half maximum). In the first level analysis, the coefficients for each contrast were estimated separately in the fixed event-related effect model. In the second level analysis, the consistency of these coefficients was estimated using the linear regression module with absolute concentrations of metabolites from 1H-MRS as the covariates.Results
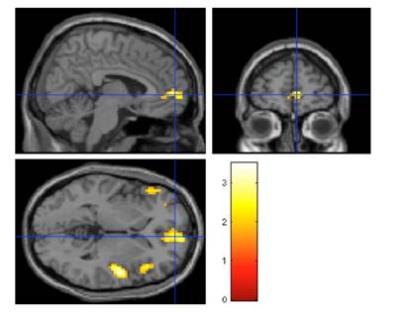
The demographic information and mean absolute concentrations of metabolites from 1H-MRS were shown in Table 1. The anterior cingulate cortex (ACC), right inferior frontal gyrus, bilateral insula and medial frontal gyrus were positively correlated with the absolute concentration of Glx ([Glx]abs) (Figure 3). Other metabolites showed no correlation with ACC activation.Discussion
Our novel finding of [Glx]abs significantly positively correlated with the activated region over the ACC provides a neurochemical basis of neural activations observed in the attention control task, i.e. numerical Stroop. As mentioned, [Glx]abs consists of both Glu and Gln, where Glu is the primary excitatory neurotransmitter, and together with its precursor, Gln, they form the Glu-Gln cycle. In other words, [Glx]abs can be deemed as a central measure of glutamatergic neurotransmission. The ACC is involved in attention control and its rich glutamatergic innervation has been reported in prior studies.2,3 As such, an elevation in this glutamatergic activity may then lead to the observed task-induced activation in the ACC.Conclusion
This is the first study which provides a neurochemical explanation of the BOLD change during an attention control task (numerical Stroop), identifying Glx as the excitatory neurotransmitter mediating the neurovascular coupling.Acknowledgements
No acknowledgement found.References
1. D'Esposito M, Deouell Y, et al. Alterations in the BOLD fMRI signal with ageing and diseases: A challenge for neuroimaging. Neuroscience 2003;4:863-872.
2. Bozkurt A, Zilles K, Schleicher A, et al. Distributions of transmitter receptors in the macaque cingulate cortex. Neuroimage 2005;25:219–229.
3. Palomero-Gallagher N, Vogt BA, Schleicher A, et al. Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Hum Brain Mapp 2008;30:2336–2355.
Figures
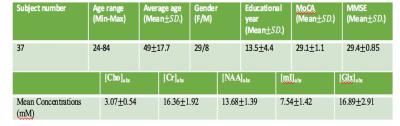
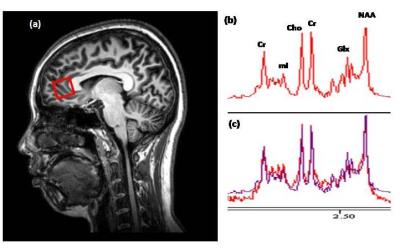
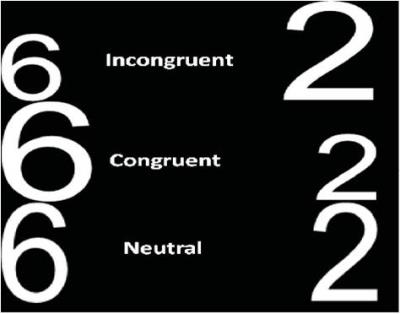
Figure 2. The numerical Stroop experimental conditions include incongruent, congruent and neutral conditions. Each subject should choose the number with higher numerical value despite of its physical size.