5380
Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy1Vanderbilt University, Nashville, TN, United States
Synopsis
Seizures in temporal lobe epilepsy (TLE) disturb brain network physiology and lead to brain connectivity disturbances. We used resting-state functional MRI (fMRI) recordings in TLE patients and controls to examine functional connectivity between brainstem ARAS structures and cortical/subcortical regions. ARAS connectivity was significantly lower in TLE patients than controls, with largest connectivity decreases noted in neocortical regions ipsilateral to the epileptogenic zone. Diminished ARAS connectivity was related to seizure frequency and neuropsychological impairments. Functional connectivity analysis of small brainstem structures using fMRI is feasible and may provide important information regarding mechanisms of disease in neurological disorders.
Purpose: Epilepsy is a devastating neurological disorder affecting approximately 1% of the population. Seizures in temporal lobe epilepsy (TLE) disturb brain network physiology and lead to brain connectivity disturbances.1, 2, 3 A perplexing question in TLE is: Why do seizures originating in a focal brain region lead to more global ictal and interictal problems, such as loss of consciousness and neurocognitive impairment, which suggest disturbance of widespread brain networks? We have previously hypothesized that recurrent seizures in TLE may lead to abnormal connections involving subcortical activating structures including the ascending reticular activating system (ARAS), contributing to neocortical dysfunction and neurocognitive impairments.3, 4 However, no prior studies of ARAS connectivity have been previously reported in epilepsy patients, and few studies have examined brainstem connectivity in neurological disorders given challenges inherent to fMRI recordings in small deep-seated regions.
Methods: We used resting-state functional MRI (rs-fMRI) recordings in 27 TLE patients and 27 matched controls to examine functional connectivity (partial correlation) between eight brainstem ARAS structures and 105 cortical/subcortical regions. We utilized a MRI-based atlas of ARAS structures recently developed by others using high angular resolution diffusion imaging together with postmortem microscopic histopathological examination of adult human brains.5 These ARAS structures included 8 pontomesencephalic brainstem regions: the cuneiform/subcuneiform nuclei (SC), dorsal raphe nucleus (DR), locus coeruleus (LC), median raphe nucleus (MR), parabrachial complex (PBC), pontine nucleus oralis (PO), pedunculopontine [tegmental] nucleus (PPN); and ventral tegmental area (VTA). We examined ARAS connectivity patterns in control subjects and TLE patients, and related connectivity relationships to disease-related parameters and neuropsychological performance across patients.
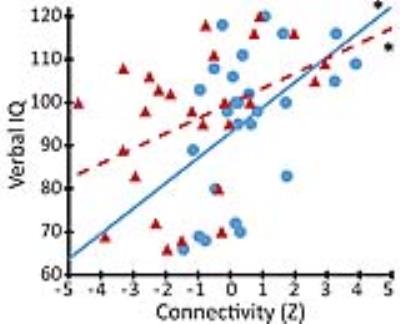
Results: Across all subjects, regions showing the most positive connectivity to ARAS structures included thalamus, limbic system, and nucleus accumbens/basal forebrain, consistent with prior studies of ARAS projections. Overall ARAS connectivity was significantly lower in TLE patients than controls (p < 0.05, paired t-test), with largest connectivity decreases noted in insular, lateral frontal, posterior temporal, and opercular cortex (Fig. 1). Diminished ARAS connectivity in TLE patients was related to increased frequency of consciousness-impairing seizures (p < 0.01, Pearson correlation), suggesting an association with severity of illness. Furthermore, reductions in ARAS connectivity were significantly associated with impairments in verbal IQ (Fig. 2), attention, executive function, language, and visuospatial memory on neuropsychological evaluation (p < 0.05, Spearman’s rho or Kendell’s tau-b).
Discussion: The present study is the first to examine ARAS functional connectivity in epilepsy patients, and relate connectivity reorganization to disease characteristics and neuropsychological parameters. Concordance between our findings in control subjects and prior studies help validate the reliability of our results. Overall, we found reduced connectivity between ARAS structures and cortical/subcortical regions in patients vs. controls. Based on our present results and prior human and animal investigations,6-8 we postulate that: i) seizures in TLE result in aberrant ictal activity in ARAS structures, ii) frequent seizures lead to interictal connectivity disturbances between ARAS and other cortical/subcortical regions, iii) diminished connectivity between ARAS and these regions may contribute to long-term reductions in neocortical metabolism, gray matter volume, and connectivity seen in epilepsy, and iv) these network disturbances may ultimately lead to neurocognitive and psychosocial problems suffered by epilepsy patients.
Conclusion: Recurrent seizures in TLE may lead to disturbances in ARAS functional connectivity, contributing to more widespread network dysfunction which may help explain neurocognitive problems suffered in this devastating disorder. Functional connectivity analysis of small brainstem structures using rs-fMRI is feasible and may provide important information regarding mechanisms of disease in neurological disorders.
Acknowledgements
This work was supported by the National Institutes of Health grants K99 NS097618 (DJE) and R01 NS075270 (VLM) and by an American Epilepsy Society/Epilepsy Foundation grant (DJE). For brain atlases used in this study, we thank the Harvard Center of Morphometric Analysis (Harvard-Oxford Atlas) and the Martinos Center for Biomedical Imaging (Harvard Ascending Arousal Network Atlas).References
1. Morgan VL, Abou-Khalil B, Rogers BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connectivity. Feb 2015;5(1):35-44.
2. Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Research. Feb 2010;88(2-3):168-178.
3. Englot DJ, Hinkley LB, Kort NS, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. May 16 2015;138(8):2249-2262.
4. Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. Aug 24 2016: in press.
5. Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. Journal of Neuropathology and Experimental Neurology. Jun 2012;71(6):531-546.
6. Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation. Cerebral Cortex. Mar 3 2016: in press.
7. Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. Feb 4 2015;85(3):561-572.
8. Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. Journal of Neuroscience. Oct 14 2009;29(41):13006-13018.
Figures