5365
Mouse BOLD fMRI imaging during operant learning at ultra-high field (14 T)1Department of Integrated Biosciences, The University of Tokyo, Tokyo, Japan
Synopsis
A setup for operant learning fMRI was developed and inserted in a horizontal bore 14 T MRI. After the habituation of head-fixed mice, visual stimulation was delivered as CS and a water as reward was supplied automatically in response to licking behavior, for an operant learning task. We analyzed fMRI data between the correct and the error trials and found the BOLD elevation of brain areas including the visual cortex and the hippocampal formation when mice performed the correct trial. Mouse BOLD fMRI in operant learning task will offer unexperienced data to basic as well as clinical research fields.
Purpose
Motivation of this study is to visualize BOLD fMRI acitivation of the neural correlate during cognitive processes, including perception and task contingencies (action-outcome rules).Introduction
In rodents, operant learning is a suitable task to assess such neural functions [1]. We applied head-fixed imaging technique of awake rodents frequently used in optical imaging [2] for BOLD fMRI imaging of awake mouse. Mouse is the most commonly used animal model and there are tremendous numbers of mutant strains with targeted gene-insertion and/or gene-knockout, which resemble many kinds of neurodegenerative as well as neuropsychological diseases, however, we need higher spatial resolution. To achieve this goal, we utilized ultra-high field MRI [3, 4] and awake rodents fMRI technologies [5, 6] to develop an fMRI setup for imaging neural correlates for operant learning.Methods
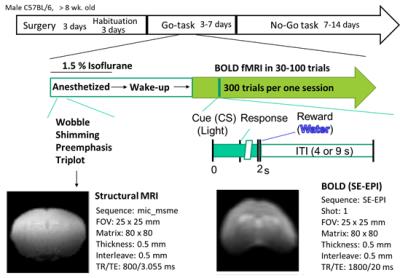
Setup: For operant learning at ultra-high field, we developed a non-magnetic chamber for operant learning, in which visual stimulation was delivered as conditioned stimulus (CS) and water (2 μl per shot) as reward. When a mouse responds to the CS by licking behavior, a tip of water was delivered automatically after the monitoring of licking behavior by a non-magnetic optic sensor. A fMRI setup (Fig 1) consists of a horizontal bore actively-shielded magnet (14 T, 46.5 mm), a hybrid gradient-shim coil (600 mT/m in x, y, z direction and 1st and 2nd order 10-layer room shim coils in 30-46 mm), and a surface coil (15 x 22 mm rectangular loop coil) used for transmission and reception, and interfaced to a ParaVision 5.1 console (Bruker BioSpin, Ettlingen, Germany).
Operant Learning fMRI: Mouse conducted an operant learning (Fig 2), in which we delivered visual stimulation as conditioned stimulus and water as reward. When a mouse respond to CS by licking behavior, we delivered a water in response to this behavior. Licking behavior was monitored by a non-magnetic optic sensor. To obtain BOLD signal during this operant reversal learning of awake mouse, we utilized Spin Echo EPI sequence (TE/TR=20/1800 ms, FA=90, FOV=25 x 25 mm, 0.32 x 0.32 x 1.0 mm). We acquired 30-100 sets of fMRI from the frontal cortex to the visual cortex of mouse. All animal procedures and experiments in this study were approved by our institution’s ethics committee and were conducted according to the guidelines for animal experimentation required by the University of Tokyo.
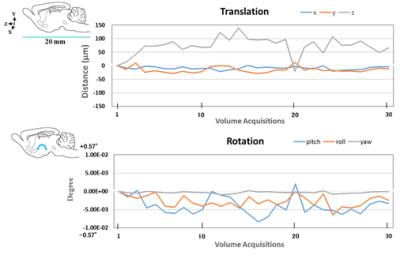
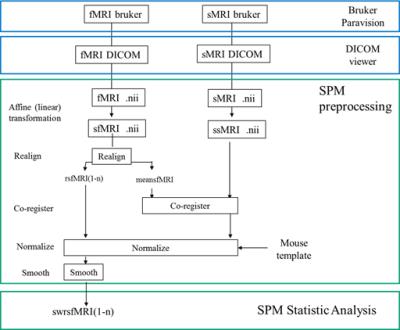
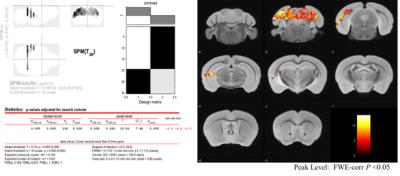
fMRI data processing: Set of fMRI data were divided into the correct and the error trials. fMRI data were processed by SPM12 attached with SPMmouse. and tiny movement of mouse head during fMRI scan were normalized by the alignment (Fig. 3). A pipeline for analyzing fMRI data was shown in Fig 4. Finally, we plotted the activation area of behaving mouse by the two-sample t-test between the correct and the error trials (FWE p<0.05).
Results
Fig. 5 shows BOLD fMRI acitivation of mouse brain during operant learning task. We statistically analyzed fMRI data between the correct and the error trials. In a representative session, there were 19 correct and 11 error trials. After FWE P < 0.05 in peak-level, we clearly detected the BOLD activation of mouse brain when performing the correct trails of operant learning. The areas include unilateral visual cortex, posterior hippocampus, and superior colliculus. These areas are essentially involved in vision and memory (Correct vs Error, FWE P<0.05).Discussion
We are in progress to setup operant reversal learning fMRI after the set-shift by using No-Go-Task (Fig. 2), which may cover other cognitive and executive functions, including attentional set-shifting, inhibitory control and decision-making [1, 6]. Mouse BOLD fMRI during operant learning will offer unexperienced data to basic as well as clinical research fields.Acknowledgements
This study was financially supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (Grant No. 25115004).References
[1] Hvoslef-Eide M, Mar AC, Nilsson SR, Alsiö J, Heath CJ, Saksida LM, Robbins TW, Bussey TJ. The NEWMEDS rodent touchscreen test battery for cognition relevant to schizophrenia. Psychopharmacology (Berl). 2015; 232: 3853-72.
[2] Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010; 464: 1182-6.
[3] Yu X, He Y, Wang M, Merkle H, Dodd SJ, Silva AC, Koretsky AP. Sensory and optogenetically driven single-vessel fMRI. Nat Methods. 2016; 13: 337-40.
[4] Ciobanu L, Solomon E, Pyatigorskaya N, Roussel T, Le Bihan D, Frydman L. fMRI contrast at high and ultrahigh magnetic fields: insight from complementary methods. Neuroimage. 2015; 113: 37-43.
[5] Ferris CF, Kulkarni P, Toddes S, Yee J, Kenkel W, Nedelman M. Studies on the Q175 Knock-in Model of Huntington's Disease Using Functional Imaging in Awake Mice: Evidence of Olfactory Dysfunction. Front Neurol. 2014; 5: 94.
[6] Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016; 351: aac9698.
Figures