5356
Multi-Echo EPI improves olfaction-related brain activation1Fraunhofer Institute for Process Engineering and Packaging (IVV), Freising, Germany, 2Diagnostic and Interventional Neuroradiology, RWTH Aachen University, Aachen, Germany, 3GE Global Research, Munich, Germany, 4GE Healthcare, Orsay, France, 5Food Chemistry and Molecular Sensory Science, Technical University of Munich, Freising, Germany, 6ZIEL Institute for Food and Health, Clinical Nutritional Medicine, Technical University of Munich, Munich, Germany, 7Institute of Nutritional Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany
Synopsis
Multi‑echo planar imaging (MEPI) was explored regarding its ability to overcome the limitations of conventional EPI imaging in studies related to olfaction. MEPI offers great sensibility even in brain regions, which are affected by susceptibility artifacts in EPI imaging. Five subjects were scanned using an event-related olfactory task with both sequences. The subsequent comparison shows that three echoes MEPI has advantages regarding olfaction-related brain activation compared to single echo EPI.
Introduction
The measurement of brain activation during sensory perception using functional magnetic resonance imaging (fMRI) is limited by magnetic field inhomogeneity in the brain areas adjacent to the nose and ears. This makes it difficult to show the primary processing steps in olfaction [1].
We, therefore, explored images acquired with a novel sequence, namely multi‑echo echo planar imaging (MEPI) for its ability to overcome the limitations of brain imaging by conventional echo-planar imaging (EPI). The combination of three echo times in MEPI can compensate signal and BOLD sensitivity losses in EPI [2–5]
Methods
Five healthy volunteers (all females) participated in our scanning session with the two different sequences. We used olfactory stimulation with two food-related odors, one attractive and one aversive, as a model approach to compare the three sequences and identify their strengths and weaknesses in the context of imaging olfactory processing. Odors were delivered using a constant-airflow olfactometer [6] during 3 seconds after an auditory sniff cue to the subjects. In the control condition the headspace of water was delivered by the olfactometer, presenting no odor to the participant. As an attractive, food-related odor we used strawberry [7], and as an aversive, food-related odor we used liver [8]. This event-related olfactory fMRI task was conducted in a GE Discovery MR750w 3T scanner with a 12-channel head coil. Two pulse sequences were used for each volunteer in a randomized order: A standard EPI and a three echoes MEPI [4]. The main sequence parameters for both sequences were, respectively: TR = 2 s / 2.5 s, TEs = 30 / 14.4, 35.5, 56.5 ms, FA = 77° / 83º, matrix = 80 x 80 / 64 x 50, FoV = 20.0 cm, slice thickness = 2.5 mm, gap = 0.5 mm, # slices = 32 / 22, ASSET = 2. Analysis of the fMRI data was conducted using SPM12 based on Matlab except for the slice time correction and realignment steps for MEPI followed by the combination of the images of different TEs (echo times), which were conducted in SPM8 [4,5]. Fixed-effects group analysis was conducted with five subjects per sequence for the condition odor versus non-odor.Results
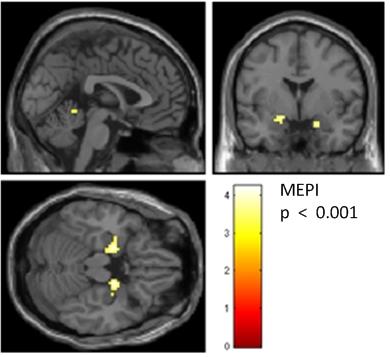
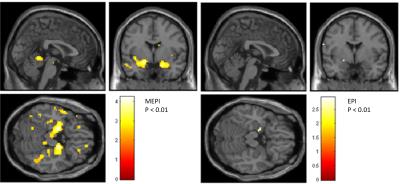
Using MEPI, activation of typical olfactory brain regions (piriform cortex) was established even with a low subject number and at a lower significance level than conventional EPI. At a significance level of p < 0.001 (uncorrected for whole-brain comparison), MEPI data of five subjects show activation of the piriform cortex while in EPI no suprathreshold clusters survived (Figure 1). For visualization purposes a lower threshold of p < 0.01 uncorrected was used. Here, activation of the piriform cortex can be shown by EPI only on the left hemisphere, while using MEPI we find stronger activation not only in bilateral piriform cortex but also bilaterally in the orbitofrontal cortex (Figure 2).Discussion
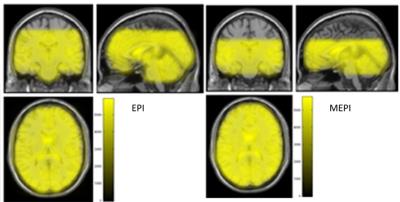
We conclude that MEPI is well suited for sensory stimulation scenarios including olfaction. This sequence is more potent to show olfaction-related brain activations than EPI, even at low number of subjects. On the other hand, MEPI cannot achieve the same brain coverage and spatial resolution as the conventional EPI even for longer TR (repetition time) (Figure 3). However, shorter readouts for MEPI could be achieved by the use of partial Fourier or higher acceleration factors for 32-channel head coil. Also, the next step could include the use of multiband (multislice) MEPI, which will allow an increase of the number of slices in the same amount of time [9]. In conclusion, we were able to establish an improvement in the visualization of olfaction-related brain activation using MEPI instead of EPI. Further optimization work should target the improvement of brain coverage to make MEPI a useful tool also in studies targeting multisensory integration processes.Acknowledgements
We thank Prof. Dr. Haase (IMETUM, TU Munich) and Dr. Schirmer (GE Global Research, Garching) for collaboration at the MRI machine and our volunteers for participation.References
[1] C. Moessnang, J. Freiherr, Olfaktorik, in: F. Schneider (Ed.), Funktionelle MRT in Psychiatrie und Neurologie, 2nd ed., Springer, Berlin, 2013. ISBN: 978-3-642-29800-4, pp. 505–521.
[2] P. Kundu, S.J. Inati, J.W. Evans, W.-M. Luh, P.A. Bandettini, Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI, NeuroImage 60 (3) (2012) 1759–1770. DOI: 10.1016/j.neuroimage.2011.12.028.
[3] O. Speck, J. Hennig, O. Speck, J. Hennig, Functional imaging by I0- and T2*-parameter mapping using multi-image EPI, Magnetic resonance in medicine 40 (2) (1998) 243–248. DOI: 10.1002/mrm.1910400210.
[4] B. Fernandez, L. Leuchs, P. Sämann, M. Czisch, V. Spoormaker, Application of multi-echo EPI on a fear conditioning task: evidence of improved BOLD detection in ventromedial prefrontal cortex: 29 (Suppl 1):S247–S400 (338), Vienna, Austria, 2016.
[5] B.A. Poser, M.J. Versluis, J.M. Hoogduin, D.G. Norris, BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI, Magnetic resonance in medicine 55 (6) (2006) 1227–1235. DOI: 10.1002/mrm.20900.
[6] J.N. Lundström, A.R. Gordon, E.C. Alden, S. Boesveldt, J. Albrecht, Methods for building an inexpensive computer-controlled olfactometer for temporally-precise experiments, International journal of psychophysiology official journal of the International Organization of Psychophysiology 78 (2) (2010) 179–189. DOI: 10.1016/j.ijpsycho.2010.07.007.
[7] P. Schieberle, T. Hofmann, Evaluation of the Character Impact Odorants in Fresh Strawberry Juice by Quantitative Measurements and Sensory Studies on Model Mixtures, J. Agric. Food Chem. 45 (1) (1997) 227–232. DOI: 10.1021/jf960366o.
[8] S. Straßer, Characterisation of the key aroma compounds in processed duck liver: Studies on their formation during roasting and differences to livers of other animal species, 1st ed., Verl. Deutsche Forschungsanst. für Lebensmittelchemie (DFA), Freising, 2012. ISBN: 978-3-938896-52-5.
[9] V. Olafsson, P. Kundu, E.C. Wong, P.A. Bandettini, T.T. Liu, Enhanced identification of BOLD-like components with multi-echo simultaneous multi-slice (MESMS) fMRI and multi-echo ICA, NeuroImage 112 (2015) 43–51. DOI: 10.1016/j.neuroimage.2015.02.052.
Figures