5355
Transient reduction in auditory midbrain responses following acute noise exposure1Department of Physics and Materials Science, City University of Hong Kong, Kowloon, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, 3Department of Radiology, Children's Hospital of Fudan University, Shanghai, People's Republic of China, 4Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong
Synopsis
Noise pollution can significantly affect sound processing, even without causing hearing loss. In this study, adult rat subjects are exposed to 100 dB sound level noise for 15 minutes. Functional magnetic resonance imaging (fMRI) and auditory brainstem response (ABR) testing with sound stimulation are performed before, 7 days after, and 14 days after exposure. ABR results show no significant threshold elevation, indicating no significant abnormalities in and near the ear. fMRI results show significant response reductions in the auditory midbrain 7 days after exposure. This suggests transient changes in central auditory gain following acute noise exposure.
Purpose
Precise hearing is crucial for survival and prosperity in a competitive world. Noise exposure from noise pollution can significantly affect sound processing in the brain even without causing hearing loss. Changes in sound processing ability are related to central auditory processing disorders (CAPDs), which affects school performance1,2 and other important functions3. In this study, we apply fMRI with sound stimulation on a rat model of acute noise exposure to study the impact on central auditory processing.Methods
Subjects were prepared for fMRI sessions similar to in our previous studies4-11. Ten normal female Sprague–Dawley rats were employed. Subjects were anesthetized with isoflurane and mechanically ventilated. Warm water was circulated underneath the subject and its vital signs were monitored (SA Instruments).
The acute noise exposure was an octave band noise (8 – 16 kHz) at 100 dB sound pressure level (SPL) lasting for 15 minutes. During exposure, the subject was unrestrained within a cage. The cage was placed below the speaker (MF1, TDT). The speaker output was calibrated in the center of the cage using a microphone (M50, Earthworks) and recorder (FR2, Fostex). After exposure, subjects were placed in standard acoustic conditions in the holding room.
Acoustic stimulation during fMRI was produced by a MF1 speaker and delivered to the ear through a custom sound tube. Pulsed tonal stimuli at 12 and 20 kHz were produced with 100% modulation depth and 50% duty cycle. The sounds were calibrated at the ear end of the tube using the M50 microphone. The stimuli were presented in a block design paradigm as in earlier studies4,8-12.
fMRI was performed before, 7 days after, and 14 days after exposure. Imaging was performed at 7 T (Pharmascan, Bruker Biospin). The fMRI sequence was: single-shot GE-EPI, FOV = 32×32 mm2, matrix = 64×64, slices = 9, slice thickness = 1.2 mm (0.2 mm gap), angle = 56°, TE = 20 ms, and TR = 1000 ms. The third slice was centered on the inferior colliculus (IC). After each time point, subjects were returned to the holding room.
Auditory brainstem responses (ABRs) were recorded at each time point. Subjects were anesthetized by sodium pentobarbital. Subdermal needle electrodes were inserted at the vertex (positive), below the pinna (negative), and at the back (ground). Click stimuli (0.1 ms) were transmitted to the recording ear. ABRs were averaged across 1024 clicks per SPL. SPL was reduced by increasing speaker–ear separation.
fMRI images were processed using standard image registration and normalization algorithms implemented in SPM (Wellcome Trust Centre). T-value maps were also computed using the general linear model implemented in SPM. Statistical comparisons were performed with repeated measures analysis of variance and Tukey’s honest significant difference test. A p-value threshold of 0.05 was defined as significantly different.
Results
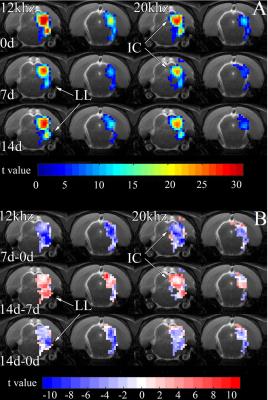
Short duration, high SPL acute noise exposure reduces fMRI responses in the IC of the midbrain 7 days after exposure. This is observed in the t-value maps in Fig. 1A, acquired before (0d), 7 days after (7d), and 14 days after (14d) exposure. By 14d, the responses recover to near pre-exposure levels. The t-value difference maps in Fig. 1B further illustrate this transient reduction.
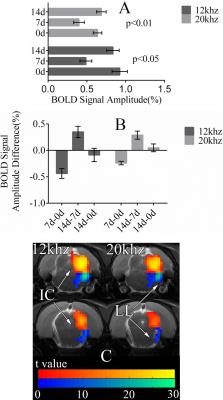
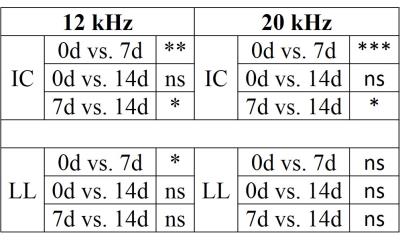
At 7d, fMRI signals from the IC during 12 kHz stimulation are 0.4% lower than 0d (Fig. 2). By 14d, signals are only 0.1% lower than 0d. During 20 kHz stimulation, signals from 7d are 0.2% lower than 0d and signals from 14d are slightly higher than 0d. Signals from 7d are statistically significantly lower than those from 0d and 14d (Fig. 3). The signals from 0d and 14d are statistically similar. In the lateral lemniscus (LL) of the midbrain, signals at 7d are significantly lower than 0d. Acute noise exposure leads to transient reductions in midbrain responses with timescale of weeks.
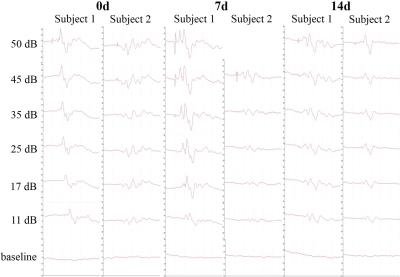
ABRs from two subjects in Fig. 4 show the lowest SPL at which clearly discernible responses are observed with our system is 11 dB at all time points. This shows that the acute exposure does not induce significant hearing threshold shifts. Therefore, the above fMRI observations likely reflect changes near the midbrain level.
Discussion and Conclusion
The results indicate a transient reduction in auditory midbrain responses one week after acute noise exposure. This change likely occurs around the midbrain level. Also, the timescale of change is before that of cochlear and auditory nerve degeneration, which occur with timescale of months13,14. Acute noise and noise pollution in general can impact our hearing in ways that go beyond conventional hearing loss15. This is an important clinical and public health question that requires further investigation.Acknowledgements
No acknowledgement found.References
1. Bellis TJ, Ferre JM. Multidimensional approach to the differential diagnosis of central auditory processing disorders in children. Journal of the American Academy of Audiology 1999;10:319-28.
2. Heine C, Slone M. The impact of mild central auditory processing disorder on school performance during adolescence. The Journal of school health 2008;78:405-7.
3. Musiek FE, Chermak GD. Handbook of Central Auditory Processing Disorder, Auditory Neuroscience and Diagnosis. San Diego: Plural Publishing; 2013.
4. Cheung MM, Lau C, Zhou IY, et al. BOLD fMRI investigation of the rat auditory pathway and tonotopic organization. Neuroimage 2012;60:1205-11.
5. Cheung MM, Lau C, Zhou IY, et al. High fidelity tonotopic mapping using swept source functional magnetic resonance imaging. Neuroimage 2012;61:978-86.
6. Gao PP, Zhang JW, Cheng JS, Zhou IY, Wu EX. The inferior colliculus is involved in deviant sound detection as revealed by BOLD fMRI. Neuroimage 2014;91:220-7.
7. Gao PP, Zhang JW, Fan SJ, Sanes DH, Wu EX. Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. Neuroimage 2015;123:22-32.
8. Lau C, Pienkowski M, Zhang JW, McPherson B, Wu EX. Chronic exposure to broadband noise at moderate sound pressure levels spatially shifts tone-evoked responses in the rat auditory midbrain. Neuroimage 2015;122:44-51.
9. Lau C, Zhang JW, Cheng JS, Zhou IY, Cheung MM, Wu EX. Noninvasive fMRI investigation of interaural level difference processing in the rat auditory subcortex. PLoS One 2013;8:e70706.
10. Lau C, Zhang JW, McPherson B, Pienkowski M, Wu EX. Long-term, passive exposure to non-traumatic acoustic noise induces neural adaptation in the adult rat medial geniculate body and auditory cortex. Neuroimage 2015;107:1-9.
11. Zhang JW, Lau C, Cheng JS, et al. Functional magnetic resonance imaging of sound pressure level encoding in the rat central auditory system. Neuroimage 2013;65:119-26.
12. Lau C, Zhang JW, Xing KK, et al. BOLD responses in the superior colliculus and lateral geniculate nucleus of the rat viewing an apparent motion stimulus. Neuroimage 2011;58:878-84.
13. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. The Journal of Neuroscience 2009;29:14077-85.
14. Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary Neural Degeneration in the Guinea Pig Cochlea After Reversible Noise-Induced Threshold Shift. Jaro-J Assoc Res Oto 2011;12:605-16.
15. Liberman MC. Hidden Hearing Loss. Sci Am 2015;313:48-53.
Figures