5353
Potential changes of cerebral perfusion alterations in sensorineural hearing loss1Jiangsu Key Laboratory of Molecular Imaging and Functional Imaging Department of Radiology, Nanjing, People's Republic of China, 2Jiangsu Key Laboratory of Molecular Imaging and Functional Imaging Department of Radiology
Synopsis
To explore the effect of hearing loss on cerebral perfusion, by using a whole brain arterial spin-labeling (ASL) MRI technique. We recruited 8 hearing loss patients and 6 healthy controls, and identified the cuneus with hypoperfusion in SHL patients which may provide new insights into SHL-associated psychological abnormalities.
Introduction
Hearing loss (HL) may cause difficulties in everyday life situations and reduce quality of life (QoL). Moreover, the prevalence of HL is high.1,2Perfusion provides oxygen and nutrients to tissues and is closely tied to tissue function, and disorders of perfusion are major sources of medical morbidity and mortality.3 The aim of this study was to assess the psychological domain of QoL and explore the effect of hearing loss on cerebral perfusion, by using a whole brain arterial spin-labeling (ASL) MRI technique.Methods
8 HL patients (mean age was 54.0 years with 3 males and 5 females), 6 healthy controls(mean age was 50.0 years with 2males and 4 femals)received pulsed arterial spin labeling (PASL) scan for measuring cerebral blood flow (CBF) and clinical assessments. Psychology assessment covering several domains was performed by an experienced neurologist blind to the group allocation using SAS and HAMD test.
ASL sequence was acquired by the following paraumeters: slice =27, repetition time (TR)=4000ms, aecho time (TE)=12ms, slice thickness=4mm,flipangle=90°, field of view=220mm×220mm, acquisition matrix =64×64, number of controls/labels =52 pairs. T1-weighted magnetization-prepared rapid gradient echo imaging (MPRAGE) sequence was acquired to facilitate functional image preprocessing: section =176, TR = 1900 ms, TE = 2.48 ms, slice thickness = 1.0 mm, flip angle =9, field of view =250 mm × 250 mm, acquisition matrix =256 × 256. Finally, fluid-attenuated inversion recovery (FLAIR) images were obtained: TR = 8500 ms, TE = 94 ms, slice =20, slice thickness = 5 mm.
CBF comparisons were processed with REST software. Statistical tests across groups were performed using a voxel-based, two-sample t-tests. AlphaSim correction based on Monte Carlo simulation algorithm was used to correct for multiple comparisons (single voxel P value = 0.05, FWHM = 6mm, with 61 × 73 × 61mm3 grey matter mask, which yielded a corrected threshold of P < 0.05, cluster size > 601 mm3, (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf)).
Results
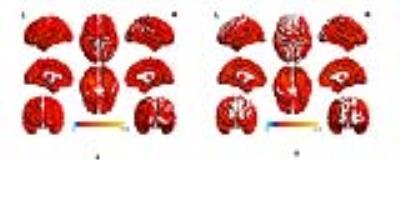
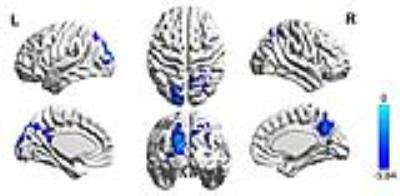
HL patients and controls were well matched for age, gender, education head motion.The average CBF corrected maps were showed in Fig.1. Fig.2 showed that group differences of CBF correct maps between sensorineural hearing loss patients and healthy controls, compared to healthy controls, SHL patients showed significantly lower CBF in the left cuneus. Table 1 showed significantly decreased CBF value in SHL patients compared with healthy controls.Discussion
As Fig 1 showed, in each group,perfusion occipital lobe, temporal lobe and the putative default-mode network regions including posterior cingulate cortex (PCC), precuneus and MPFC were higher than other regions, which is consistent with previous results.4
People suffering from hearing disturbances show a low level of life quality,5 anxiety and depression symptoms6 and a serious deficit of memory capacity .7As previously reported, the cuneus is believed to have a variety of cognitive functions including in working memory, cognitive control and behavioral engagement.8,9,10 What’s more, the cuneus is found to be related with psychological symptoms recent years.11 Now we haven’t found any correlation between CBF of cuneus and psychological symptoms, it’s the limitation of sample size, we are trying to recruit more people.
Taken all, we inferred that sensorineural hearing loss might cause psychological symptoms. To our knowledge, this is the first study that have identified cuneus abnormalities in sensorineural hearing loss patients by using ASL perfusion MRI. we identified brain regions with hypoperfusion in SHL patients, especially the cuneus, which is the important structure that often show vulnerability in the early process of neurode generation.
Conclusion
Our findings of cerebral hypoperfusion might provide valuable insights into the neural substrate of SHL-associated psychological impairments.Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China ( No.81520108015 ) .We thank Xu Feng and Jing Zhao, Department of otorhinolaryngology, Affiliated Zhongda Hospital of Southeast University, for their assistance with the data collection.References
1. Soo, Y. et al. Disability of Hearing Impairment Is Positively Associated With Urine Albumin / Creatinine Ratio in Korean Adults : The 2011 – 2012 Korea National Health and Nutrition Examination Survey. 2016, 9, 212–219 .
2. Polku, H. et al. Hearing and Quality of Life Among Community-Dwelling Older Adults. 2016, 0, 1–10 .
3. John A. Detre, Hengyi Rao, Danny JJ Wang, et al. Applications of Arterial Spin Labeled MRI in the Brain. J Magn Reson Imaging. 2012 May , 35(5) : 1026–1037.
4. Liang, X., Zou, Q., He, Y., & Yang, Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceeding National Academy Science United State of America, 110(5), 1929-1934.
5. Mulrow, C.D., Aguilar, C., Endicott, J.E., Tuley, M.R., Velez, R., Charlip, W., Rhodes, M.C., Hill, J.A. and De Nino, L.A. Quality- of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990; 113: 188-194.
6. Jones, D.A., Victor, C.R. and Vetter, N., Hearing difficulty and its psychological implication for the elderly, J. Epidemiol. Comm. Health, 1984, 38, 75-78.
7. Peters, C.A., Potter, J.F. and Scholer, S.G., Hearing impairment as a predictor of cognitive decline in dementia, J. Am. Geriatr. Soc., 1988; 36, 981-986.
8. Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Hum Brain Mapp. 2010.
9. Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Psychopharmacol. 2008; 22 : 138–143.
10. Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp. 2011.
11. Whitford TJ, Wood SJ, Yung A, et al. Structural abnormalities in the cuneus associated with Herpes Simplex Virus (type 1) infection in people at ultra high risk of developing psychosis. Schizophr Res. 2012 Mar; 135(1-3) : 175-80.
Figures