5351
Modality specific thalamic activations in rat brain by fMRI1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Quantitative Neuroscience with Magnetic Resonance, Yale University, New Haven, CT, United States, 3Magnetic Resonance Research Center, Yale University, New Haven, CT, United States, 4Biomedical Engineering, Yale University, New Haven, CT, United States
Synopsis
The thalamus is a crucial node in cortical-subcortical circuits important for human emotion, cognition, and memory. While invasive studies in animals have revealed rich anatomical and functional separation of various thalamic nuclei, we sought to parse the different portions of the rat thalamus in relation to tactile (forepaw, whisker) and non-tactile (visual, olfactory) stimuli by high field fMRI (11.7T). We reproducibly detected BOLD activations of VPL, VPM, POM, dLGN, and MDT, where MDT activation is a novel indication of this structure’s involvement during olfactory processing. These results have significance in understanding the role of both cortical-subcortical circuits during sensory integration.
PURPOSE: The thalamus is a crucial node in cortical-subcortical circuits important for human emotion, cognition, and memory1 . Invasive work in animals and post-mortem investigations have revealed the rich anatomical and functional specificity of the thalamus. Thalamic activations have not been consistently reported for fMRI studies. With the advance of higher field strengths for fMRI approaches, better spatial resolution with higher S/N ratio is now available. The goal of the present work is to parse the different portions of the rat thalamus in relation to tactile (forepaw, whisker) and non-tactile (visual, olfactory) stimuli by high field fMRI (11.7T).
METHODS: Adult male Sprague-Dawley rats (200-250g) were used. Forepaw and whisker experiments were performed under α-chloralose (46±4 mg/kg/hr), whereas visual and olfactory experiments were performed under urethane (1.3 g/kg, b.w) anesthesia. Forepaw stimulation (2mA, 0.3 ms, 3Hz), whisker stimulation (4Hz, air puff), visual stimulation (8Hz and 1Hz, blue light) and olfactory stimulation (methyl valerate, ethyl butyrate, isoamyl acetate) were achieved using custom built hardware2. All fMRI data were obtained on a modified 11.7T Bruker horizontal-bore spectrometer (Billerica, MA) using a 1H resonator/surface coil RF probe.
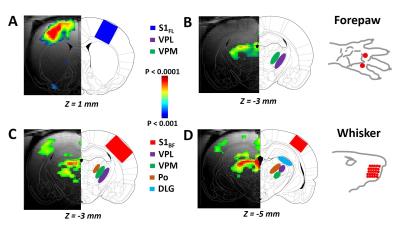
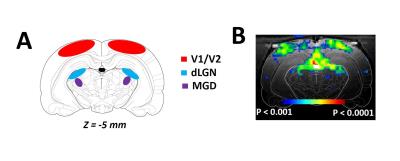
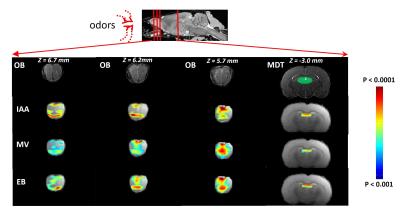
RESULTS: Our results demonstrate reproducible thalamus and cortical activity during tactile (forepaw, whisker), and non-tactile (visual, olfactory) sensory stimulation in anesthetized rats. Forepaw stimulation activated contralateral somatosensory forelimb area (S1FL) (Fig.1A), the ventral posterior lateral (VPL) and ventral posterior medial (VPM) parts of the thalamic nucleus (Fig.1B). Whisker stimulation activated contralateral somatosensory whisker barrel area (S1BF) and broader regions within the thalamus: ventral posterior medial (VPM), posterior medial (POM) and dorsal and medial parts of the lateral geniculate nucleus (Fig.1C-D). Visual stimulation activated broader regions of the visual cortex (V1 and V2) and dorsal lateral geniculate nucleus (dLGN) of the thalamic regions (Fig.2). Simultaneous imaging of olfactory bulb and the brain with high temporal resolution showed evoked BOLD responses of whole olfactory bulb and higher olfactory structures including the mediodorsal thalamic nucleus (MDT) (Fig.3). MDT responses to different odors were highly reproducible and this is the first fMRI study in rodents to show thalamic activation.
DISCUSSION: In the present study, we demonstrated a clear modality specific cortical and thalamic activation in response to somatosensory, visual and olfactory stimuli. We reproducibly detected BOLD activations of VPL, VPM, POM, dLGN, and MDT, where MDT activation is a novel indication of this structure’s involvement during olfactory processing. Thalamus is a key crossroad structure involved in various functions relative to visual, auditory, gustatory, and somatosensory senses. In all senses except for the olfactory sense, the thalamus is involved in many functions ranging from basic sensory information processing3-4 to more complex functions, such as sensory gating and attention modulation5, control of sleep states6, multisensory integration7 and memory processing8. Because of the specific organization of the olfactory pathway (i.e., no direct thalamic relay between sensory neurons and primary cortex), olfactory thalamus was often overlooked in understanding olfactory function, especially in nonhuman animal models9. Here, we provide evidence that updates the implication of the thalamus in olfactory processing. Current understanding about BOLD signal and the underlying neurophysiology is based predominantly on functions of the cerebral cortex. BOLD activations of thalamic regions, in contrast, are hard to detect because of low sensitivity and/or difficult access. To study interactions between cortical and thalamic regions by fMRI, the BOLD signal in thalamus must be measured with the same level of MR sensitivity as that achieved in cortex. Thus to establish early foundations for cross modal sensory studies, we need to measure reproducible BOLD activation patterns of thalamus for different sensory paradigms in relation to the activated cortical areas. These experiments should provide insights into understudied interactions between cortical/thalamic areas and provide a mechanistic basis to understand thalamo-cortical activity. Cortical activations are usually interpreted to represent behavior. However function of thalamic areas can be considered to be “coupled” with activities of the cerebral cortex. These results have significance in understanding the role of both cortical and thalamic areas during multisensory integration. Some studies provide evidence of thalamic influence on multisensory information processes in rats10 and humans11.
CONCLUSION: Thalamic regions are difficult to detect because of low sensitivity and/or difficult access. We can apply high field fMRI (high S/N ratio) to study thalamo-cortical as well as their reciprocal interactions. Future studies of high-resolution neuroanatomy (e.g., DTI) can provide the morphological basis of the high-resolution functional parcellation of the rat thalamus. These results have significance in understanding the role of both cortical-subcortical circuits during sensory integration.
Acknowledgements
Supported by grants from National Institutes of Health (R01 MH-067528,P30 NS-52519).References
1. Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology.1996; 76:1367–1395.
2. Sanganahalli BG, Bailey CJ, Herman P, et al, Tactile and non-tactile sensory paradigms for fMRI and neurophysiologic studies in rodents. Methods Mol Biol. 2009; 489:213-42.
3. McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol 1994; 4: 550–556.
4. Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009; 19: 408–414.
5. Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol.1998;55: 343–361
6. Steriade M. Basic mechanisms of sleep generation. Neurology. 1992;42: 9–17
7. Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;4:255
8. Jankowski MM, Ronnqvist KC, Tsanov M, et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci 2013; 7: 45.
9. Shepherd GM. Perception without a thalamus: how does olfaction do it? Neuron.2005; 46:166–168. 10. Komura Y, Tamura R, Uwano T, et al, Auditory thalamus integrates visual inputs into behavioral gains. Nat Neurosci 2005; 8:1203-9.
11. Baier B, Kleinschmidt A, Muller NG. Cross-modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. J Neurosci 2006; 26:12260-5.
Figures