5349
Dexmedetomidine-induced CBF changes measured with Arterial Spin Labeling1Neurology, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Neurology, University of Pennsylvania, PA, United States, 4Anesthesiology, University of Pennsylvania, PA, United States
Synopsis
Dexmedetomidine infusion was used to induce pharmacological unconsciousness akin to natural non-REM sleep, and brain resting-state activity changes were monitored using Arterial Spin Labeling and EEG recordings.
Our results confirm that functional changes associated with non-REM sleep in both centrencephalic and cortical structures can be monitored with ASL, revealing that selective regional CBF decreases are associated with the loss of consciousness, most significantly in the reticular activating system. The thalamic/cortical GM CBF ratio was also found to be a reliable marker of conscious state.
Introduction
Characterizing changes in human brain function during sleep is a topic of deep interest to the neuroscience community. While the encephalogram (EEG) is the most common tool in sleep research, EEG recordings are in general restricted to cortical activity and suffer from limited spatial resolution compared to other imaging methods.
We used dexmedetomidine infusion, which selectively targets pre-synaptic α2-adrenergic receptors in the locus coeruleus, to induce pharmacological unconsciousness akin to natural non-REM sleep1, and studied the brain resting-state activity changes using Arterial Spin Labeling2 (ASL). ASL is a non-invasive perfusion MRI technique, analogous to H215O-PET, that can measure cerebral blood flow (CBF), which is known to be tightly linked to neural activity through neurovascular coupling. Repeated ASL scans alternated with EEG recordings during baseline and dexmedetomidine-induced unconsciousness states, and the effect of state on CBF was assessed regionally and at the voxel-level.
Methods
14 subjects (9F, mean age=29.3±5.4 years) enrolled and 12
completed the study, after signing written informed consent. Screening
procedure included a full history and physical exam performed by a
board-certified attending anesthesiologist, a urine toxicology screen and
pregnancy test.
Subjects fasted for 8h prior to the study. Subject safety was ensured through constant vital signs monitoring and supplemental nasal oxygen administration. After the baseline scans, dexmedetomidine was loaded at 1mcg/kg via an intravenous catheter over 10min, followed by a 0.5-1.0mcg/kg/hr infusion. Pharmacological unconsciousness was confirmed by lack of response to command.
The study was conducted on a 3 Tesla Prisma Siemens system using a 64-channel head array. The complete schematic of the data acquisition is shown in Fig.1. In this work, we focused on the ASL acquisitions.
Three 4min ASL scans were distributed along each baseline and unconscious state. ASL data were acquired with a single-shot background-suppressed pCASL3,4 sequence, with 3D RARE Stack-Of-Spirals readout accelerated in the partition-encoding direction5 (labeling/PLD=1.5/1.5s, 30 label-control pairs, TR=4s, R=2, whole-brain FOV, 3.75mm isotropic resolution).
Each ASL scan was followed by a 2min EEG recording for conscious state evaluation. Subjects were also instructed to squeeze the alarm ball after the EEG recording and their response was registered. EEG was also recorded during the drug infusion period.
Images were processed using FreeSurfer5.3.0, FSL5.0.8, SPM8 and custom Matlab scripts. ASL images were realigned6, registered to the anatomical image, pair-wise subtracted, and averaged to derive a mean CBF map per series7. Final maps were smoothed (FWHM=8mm) and registered to the MNI152 template for group-level analysis.
Regional CBF was measured inside L/R cortical GM and thalamus, using FreeSurfer automatic segmentation tool. The thalamus/cortical GM ratio was then computed as $$$\frac{Thalamus_L+Thalamus_R}{Cortical GM_L+Cortical GM_R}$$$.
Differences between baseline and unconscious states were evaluated both regionally and voxel-wise using paired t-tests, after averaging the measurements within-state. For voxel-wise group assessment, maps were normalized to the average baseline map to control for inter-subject differences.
Results and discussion
EEG data analysis confirmed non-REM state after drug infusion in 10 subjects. Two subjects had corrupted data and could not be evaluated.
Thalamus-GM ratio values are displayed in Fig.2a. This CBF ratio was significantly higher during baseline (p=0.00028). Some variability was observed across subjects, as reflected in the SD bars of Fig.2, likely related to slight between-subject response variations to anesthesia.
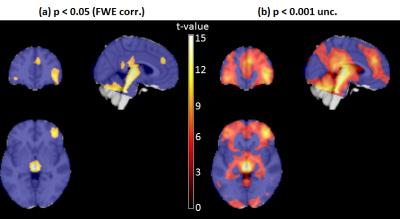
Voxel-wise CBF decreases associated with anesthesia-induced unconsciousness were also found (Fig.3). A highly significant CBF reduction was observed in the reticular activating system, starting from the locus coeruleus and extending up to medial thalamus. CBF decreases were also found in cerebellum, posterior/mid/anterior cingulate cortex, and inferior orbitofrontal cortex. The uncorrected results extended the pattern of CBF decreases in both the default-mode and fronto-parietal regions, as well as hypothalamus (Fig.3b).
These results are in very good agreement with a recent study performed with both 18F-FDG-PET and ASL, also using dexmedetomidine to induce sleep8. Although the brainstem was not included in that study, similar work conducted with H215O-PET reported thalamic and brainstem activations (including locus coeruleus) when recovering consciousness during dexmedetomidine infusion9, and cerebellar and pons hypoactivity has also been found in slow-wave sleep compared to wakefulness10.
Conclusion
This study confirms that functional changes associated with non-REM sleep in both centrencephalic and cortical structures can be monitored with ASL after infusion of dexmedetomidine, revealing that selective regional CBF decreases are associated with loss of consciousness. The most significant reduction in resting brain function was found in the reticular activating system. The thalamic/cortical GM CBF ratio was also found to be a reliable marker of conscious state. Future work will assess neural correlates between CBF and EEG markers in these areas, including delta power and frequency of sleep spindles.Acknowledgements
NIH grants UL1TR001878 and EB015893.References
1. Maze M, Regan JW. Role of signal transduction in anesthetic action. Alpha 2 adrenergic agonists. AnnNYAcadSci. 1991;625:409-422.
2. Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37-45.
3. Dai W, Garcia DM, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488-1497.
4. Vidorreta M, Wang Z, Rodríguez I, Pastor MA, Detre JA, Fernández-Seara MA. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage. 2012;66C:662-671.
5. Vidorreta M, Chang Y V., Fernández-Seara MA, Detre JA. Single-Shot Whole-Brain Background-Suppressed pCASL MRI with 1D Accelerated 3D RARE Stack-Of-Spirals Readout. In: Proceedings of the International Society of Magnetic Resonance in Medicine. Vol 23. Toronto; 2015:269.
6. Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imaging. 2012;30(10):1409-1415.
7. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116.
8. Akeju O, Loggia ML, Catana C, et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;3:e04499.
9. Langsjo JW, Alkire MT, Kaskinoro K, et al. Returning from Oblivion: Imaging the Neural Core of Consciousness. J Neurosci. 2012;32(14):4935-4943.
10. Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173-1197.
Figures
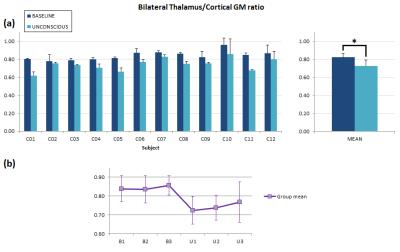
Figure 2. Regional CBF analysis.(a) Mean thalamus/cortical GM ratio values per subject obtained during the baseline and unconscious states (error bars represent SD within-state). Significant differences were found at the group level (p<0.001). (b) Evolution of thalamus/cortical GM ratio during the study. For each time-point, values are averaged across subjects (error bars represent SD). Baseline (B) state shows a higher, sustained CBF ratio compared to the unconscious (U) state, in which some heterogeneity can be observed across time, represented in the extent of the error bars, likely due to inter-subject variability in the response to the drug.