5348
Altered hemodynamic response in visual cortex observed in high-school American football players1Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 2The Translational Research Center for TBI and Stress Disorders, VA Boston Healthcare System, Boston, MA, United States, 3Purdue University, West Lafayette, IN, United States
Synopsis
There is growing concern that subconcussion could have an effect on the neural health of contact sports athletes. The hemodynamic response was measured and changes were observed in high school football athletes after exposure to impacts.
Introduction
Researchers have begun to reevaluate the effect of both concussion and sub-concussion on long-term neural health. Much of this has been focused on concussion, but research has suggested that sub-concussion (mechanical insult that does not result in readily observable symptoms) may be responsible for long-term neural deficits.1,2 The asymptomatic nature of sub-concussion makes detection of neurologic changes difficult. By conducting fMRI scans on asymptomatic high-school football players, the hemodynamic response (HDR) can be measured and tested for subtle changes in the presence of repetitive, sub-concussive head trauma.Methods
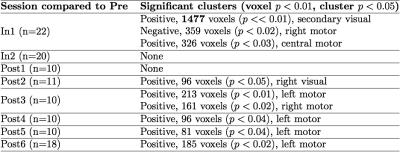
Twenty-two male high school football players (ages 14-18; avg = 16.2) were recruited for this study. Subjects participated in one scanning session before the season (Pre, n=22), two during the season (In1, n=22; In2, n=20), and up to six after the season (Post1, n=10; Post2, n=11; Post3, n=11; Post4, n=10; Post5, n=10; Post6, n=18). During each scan session, a single fMRI run was conducted (TR/TE = 500/26 msec; 10 oblique slices; FOV = 20cm; matrix = 64x64; slice thickness = 2.5mm) in which subjects were instructed to perform a rapid bimanual finger-tapping task (fingers to thumb) in response to a two-second presentation of a flashing checkerboard (nine presentations per run; jittered separation of 28-30 sec).
Preprocessing, including de-obliquing, time-shift correction, volume registration, and spatial normalization, was performed using AFNI.3 AFNI was also used to estimate the HDR using piecewise linear interpolation.
For analysis, several metrics were extracted and tested, including area-under-the-curve (AUC), time-to-peak, and timecourse standard deviation. Note that the AUC was calculated for the first ten seconds of activation, corresponding the to the expected region of positive BOLD response. Paired testing was done for each In and Post session compared to Pre, using AFNI’s 3dttest++. Multiple comparison correction was performed using AFNI’s nonparametric cluster simulation procedure.4
Results
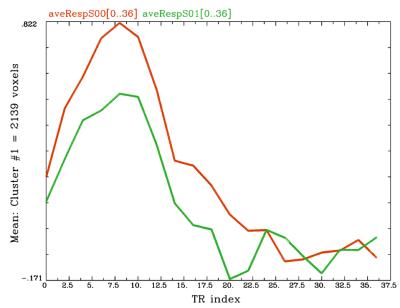
A summary of results can be found in Table 1. A significant difference in AUC was found when comparing Pre with In1. A cluster (1477 voxels) in visual cortex showed a significant (p<<0.01) drop in AUC (see Figure 1). When comparing timecourse standard deviation, one weakly significant cluster (62 voxels, voxelwise p<0.01, cluster p<0.04) was observed at In1, relative to Pre, in the visual cortex, exhibiting a decrease in variance. No difference was observed in time-to-peak, relative to Pre.Discussion
The change in HDR AUC in the visual cortex is consistent with a previous observation in older adults, relative to younger adults.5 Therefore, the change observed here might be suggestive of changes in the cortex consistent with those associated with aging, somehow here occurring during the beginning of the season’s activities. Possible short-term causes could include decreased ability to signal for increased delivery of glucose/oxygen, decreased ability of the vasculature to respond to energy needs, or a depressed metabolic state.
Previous animal studies of concussion suggest that injury is initially followed by a period of hypermetabolism that ultimately transitions to an extended period of hypometabolism.6 Given the lack of a diagnosed injury in the present athletes—i.e., no evidence of symptoms—it seems likely that the hypermetabolic stage is not being experienced, but rather the athletes are in fact exhibiting a hypometabolic condition. It could be that the repetitive mechanical insult is being partially mitigated by the brain’s natural compensatory mechanisms even though a clinically-relevant injury has not occurred. Therefore, while these athletes do not show functional deficits, there could be a greater risk of injury should the (on-going) healing processes become overloaded. This is especially a concern for younger athletes as they have been shown to recover more slowly from mTBI.7
Conclusion
Changes in the hemodynamic response suggest that repetitive sub-concussive head trauma can alter brain function. While the severity of this alteration is unclear, the brain gives evidence of on-going compensatory mechanisms even though athletes are asymptomatic. It is possible, therefore, that sufficient rest and careful monitoring of player exposure may permit sports such as football to be played with minimal increased risk of neural injury.Acknowledgements
This project was funded by the Indiana State Department of Health's Brain and Spinal Chord Injury Fund.References
1. Bailes, J. E., Petraglia, A. L., Omalu, B. I., Nauman, E., & Talavage, T. (2013). Role of subconcussion in repetitive mild traumatic brain injury: a review. Journal of neurosurgery, 119(5), 1235-1245.
2. Broglio, S. P., Eckner, J. T., Paulson, H. L., & Kutcher, J. S. (2012). Cognitive decline and aging: the role of concussive and subconcussive impacts. Exercise and sport sciences reviews, 40(3), 138.
3. Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research, 29(3), 162-173.
4. Cox, R. W., Reynolds, R. C., & Taylor, P. A. (2016). AFNI and clustering: false positive rates redux. bioRxiv, 065862.
5. Buckner, R. L., Snyder, A. Z., Sanders, A. L., Raichle, M. E., & Morris, J. C. (2006). Functional brain imaging of young, nondemented, and demented older adults.
6. Yoshino, A., Hovda, D. A., Kawamata, T., Katayama, Y., & Becker, D. P. (1991). Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper-and subsequent hypometabolic state. Brain research, 561(1), 106-119.
7. Field, M., Collins, M. W., Lovell, M. R., & Maroon, J. (2003). Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. The Journal of pediatrics, 142(5), 546-553.
Figures