5347
Dyslexia therapy customization based on dorsal-ventral pathway representation1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, India, 3Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Reading necessitates skill mastering of phonological (sound to letter), orthographic (knowledge of letter identities, position), and semantic (words meaning) processing requiring optimal interface of ventral-dorsal routes. Dyslexia, a developmental reading disorder, is an umbrella term with heterogeneity of behavioral deficits constrains the management efficiency. Persistent deficits lead to emotional, academic, social consequences necessitating evidence-based interventions. The study was planned on neurobiological-model to customize the therapeutic management. Dorsal pathway (BOLD activation) reorganization associated with improvement in reading rate, accuracy, spelling and writing flow suggest neurobiological normalization in dyslexics observed post-remediation on comparing therapy group with non-therapy and age-matched typical readers.
Purpose
Dyslexia is neuro-developmental, specific learning disorder with deficits of phonological awareness, processing speed, working memory, executive function and orthographic decoding abilities, leading to reading difficulties1-3. It is a heterogeneous condition with variability in manifestations of deficits4,5 where the cortical areas or their connections may help us to understand the phenomenon6. Rehabilitative measures are not generally based on basic neurobiological principles, despite evidence from animal models, anatomical and in-vivo human brain reorganization6,7. Thus, in this study, interventional programs are customized evidence based, clinical performance, and functional MRI (fMRI), for efficient outcome measures8.Introduction
Task based fMRI gives visible and quantifiable neurobiological difference(s) associated with specific psychological symptoms in dyslexia, where inferior frontal cortex of dorsal stream reveals cortical signature for monitoring behavioral remediation6,9. Hypothesis was made to plan the management based on these neuro-functional differences for optimal normalization and to observe the outcome by behavioral and brain measures.Methodology
Study was randomized controlled, with therapy (Rx group, n = 12, mean age: 11.38±2.41 years) and non-therapy (nonRx group, n = 12, mean age: 11.09±2.71 years) dyslexic groups compared with age-matched (n=12, 12.06±2.33 years) healthy control children. Remediation consisted of 28-30 sessions (6 months), techniques based on the neurobiological approach drilled for multiple times (Hebbian principle)10-14. Therapeutic targets, namely, (i) visual training for focusing, attentional filtering, inhibition of distracters, grapheme discrimination; (ii) auditory training for phonological awareness; (iii) kinesthetic-sensory-motor articulation training for phonological errors; (iv) working memory and executive control; (v) language enrichment at lexical, semantic and syntactic levels; (vi) generalization to reading, were prioritized/chosen based on BOLD activation as positive and negative signs. Baseline and post-therapy investigations included clinical assessment and task based fMRI (phonological, lexico-semantic and syntactic processing) acquired with 32 channel head coil in 3T MR scanner (Achieva M/s. Philips HealthCare, The Netherlands); using EPI with parameters echo train length 33, TR 2 s. Data were processed in SPM12. The visual tasks were presented using E-Prime (version 1.1, Psychology Software Tools Inc, USA) on a LCD monitor (ESys fMRI System, Invivo Corp, M/s. Philips Healthcare). Phonological (word-pseudowords judgment) and lexico-semantic (abstract-concrete nouns) tasks consisted of 222 dynamics and syntactic (simple sentences) task, 90 dynamics. Clinical data was analyzed using SPSS (version 17, SPSS Inc., IBM Business Analytics).Results and Discussion
At baseline, in typical readers (age-matched healthy control) the fast-guess inhibition mechanism for pseudowords (nonwords) was observed in comparison to meaningful words and the lexico-semantic judgment showed top-down processing in clinical performance and BOLD activation. In dyslexics, the inhibitory fast-guess mechanism for nonwords was non-differentiable and orthographic-phonological (O<=>P) BOLD activation during meaningful judgment was modified (top-down processing). Ventral lexico-semantic pathway was involved while processing abstract nouns in dyslexic and healthy control children, whereas dorsal-route executive control was modified in dyslexics as compared to controls, in concordance with literature6,15,16. The ventral regions are attributed to integrating bottom-up processing (feed-forward) and dorsal route processing influence reading (irrespective of task) by top-down gating, but the strength is modulated by task and attention15. Post diagnosis as dyslexic, the children were randomly allocated into two groups (Rx and nonRx groups) following CONSORT 2010 and post-hoc group analysis (Bonferroni test) showed statistically non-differentiable difference at baseline, delineating appropriate randomization.
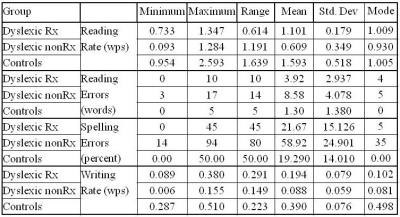
Post-therapy changes were assessed with repeated measure ANOVA for clinical performance (summarized in figure 1, table 1) and with ANOVA for BOLD activation (Figures 2, 3), which show the statistical significant difference in clinical performance and changes during phonological, lexico-semantic and syntactic processing suggestive of improvement at behavior level (reading rate 0.609 words per second, spelling errors <25%, rate of written expression 0.194 words per second) and BOLD reorganization in dorsal-ventral interface (including frontal-parietal and temporo-occipital areas; BA 6, 9, 10, 11, 44, 45, 47, 13, 21, 22, 37, 39, 17, 18, 19 and BA 31, 32) attributing to top-down executive control of dorsal route. Thus, planning remediation strategies based on brain measures along with behavioral performance optimize the improvement as normalization rather than compensatory changes 1,6,10, 11.
Acknowledgements
No acknowledgement found.References
[1] Horowitz-Kraus T, Toro-Serey C, DiFrancesco M, 2015. Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PLoS ONE, 10(7); e0133762.
[2] Shaywitz SE, Shaywitz BA, 2008. Paying attention to reading: The neurobiology of reading and dyslexia. Dev. Psychopathol., 20; 1329-1349.
[3] Norton ES, Beach SD, Gabrieli JDE, 2015. Neurobiology of Dyslexia. Curr Opin Neurobiol.; 0: 73-78.
[4] Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ, 2012. Grey Matter Alterations Co-Localize with Functional Abnormalities in Developmental Dyslexia: An ALE Meta-Analysis. PLoS ONE 7(8); e43122.
[5] Gori S, Facoetti A, 2014. Perceptual learning as a possible new approach for remediation and prevention of developmental dyslexia. Vision Res., 99; 78-87.
[6] Eden GF, Olulade OA, Evans TM, Kranick AJ, Alkire DR, 2016. Developmental dyslexia. In Hickok G, Small SL (Editors), Neurobiology of language. Academic Press Elsevier: San Diego, CA, 2016; pp 815-822.
[7] Small SL, Flores DK, Noll DC, 1998. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain And Language 62, 298–308.
[8] Reid G, 2016. Explaining dyslexia: the range of research. In: Reid G (Editor) Dyslexia: A Practitioner's Handbook (5th Edition). Wiley Blackwell: West Sussex, UK, 2016 pp 17-34.
[9] Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, et al., 2013. Cortical Signatures of Dyslexia and Remediation: An Intrinsic Functional Connectivity Approach. PLoS ONE, 8(2): e55454.
[10] Garcia-Madruga JA, Gomez-Veiga I, Vila JO, 2016. Executive functions and the improvement of thinking abilities: the intervention in reading comprehension. Frontiers in Psychology, 7; 58.
[11] Ylinen S, Kujala T, 2015. Neuroscience illuminating the influence of auditory or phonological intervention on language-related deficits. Frontiers in Psychology Cognitive Science, 6; Article 137; 1-9.
[12] Garagnani M, Wennekers T, Pulvermuller F, 2008. A neuroanatomically grounded Hebbian-learning model of attention-language interactions in the human brain. European J Neuroscience, 27; 492–513.
[13] Nelson CA, 2000. The neurobiological basis of early intervention. In: Shonkoff JP, Meisels SJ (Editors) Handbook of early intervention (2nd edition). The Press Syndicate of the University of Cambridge: Cambridge University Press, UK, 2000 pp 204-227
[14] Hebb DO, 1949. The organization of behaviour: A neuropsychological theory. John Wiley: New York, 1949.
[15] Price CJ, 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62; 816-847
[16] Zhou W, Xia Z, Bi Y, Shu H, 2015. Altered connectivity of the dorsal and ventral visual regions in dyslexic children: a resting-state fMRI study. Front. Hum.Neurosci., 9; 495
Figures