5326
Value of frequency domain resting state fMRI metrics ALFF & fALFF in the assessment of brain tumor induced neurovascular uncoupling1Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
In brain tumor patients, coupling between neuronal activity and BOLD response is often disrupted (known as neurovascular uncoupling (NVU)), resulting in dangerous underestimation of true extent of eloquent cortex in pre-surgical planning. With increasing popularity of resting state fMRI (rsfMRI) for presurgical mapping, it becomes critical to investigate effects of NVU in rsfMRI. A recent study demonstrated that tumor-related NVU can impact resting state functional connectivity within the sensorimotor network as assessed using a seed-based correlation analysis (SCA).2 We now explore whether NVU may also affect the rsfMRI frequency domain metrics ALFF (amplitude of low-frequency fluctuation) & fALFF (fractional ALFF).
Purpose
Neurovascular uncoupling (NVU) is an under-recognized limitation of clinical BOLD fMRI. In patients with brain tumors or other focal brain structural lesions, the coupling between neuronal activity and the subsequent BOLD response is often disrupted, resulting in dangerous underestimation of true extent of eloquent cortex in pre-surgical planning.1 With recent growing interest in using resting state BOLD fMRI (rsfMRI) for presurgical mapping, the investigation of the effects of NVU on rsfMRI becomes more critical. A recent study,2 demonstrated that brain tumor-related NVU can impact resting state functional connectivity within the sensorimotor network as assessed using a seed-based correlation analysis (SCA) similar to previously published findings of task-based fMRI (tbfMRI)3. We wished to explore whether NVU may also affect the resting state fMRI frequency domain metrics ALFF (the amplitude of low-frequency fluctuation4) & fALFF (fractional ALFF5).Methods
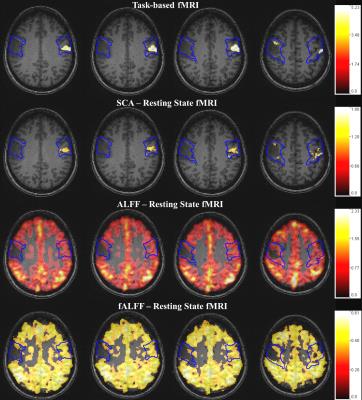
Twelve de novo brain tumor patients who underwent clinical fMRI exams including tbfMRI and rsfMRI on 3T MRI systems were included in this IRB-approved study. Each patient displayed decreased/absent tbfMRI activation in the primary ipsilesional sensorimotor cortex in the absence of corresponding motor deficit or suboptimal task performance, consistent with NVU.3 Imaging was performed on a 3.0 T Siemens Trio MRI with a 12-channel head matrix coil using a 3D T1 MPRAGE (TR=2300 ms, TI= 900 ms, TE= 3.5 ms, 9° FA, 24-cm FOV, 256x 256x176 matrix, slice thickness 1 mm) for structural imaging and multiple 2D GE-EPI T2* weighted BOLD sequences for both task & resting functional imaging (TR=2000 ms, TE=30 ms, 90° FA, 24-cm FOV, 64x64x33 matrix, 4 mm slice thickness with 1 mm gap between slices, interleaved acquisition). 180 volumes were acquired in 6 minute long rsfMRI scan. A vertical tongue movement task and a bilateral simultaneous sequential finger tapping task (each 3 minutes duration with alternating 30 second blocks of movement and rest) were used for tbfMRI. Instructions for all tasks were visually cued. SPM12 was used for preprocessing of tbfMRI & rsfMRI data (slice timing correction, realignment, normalization to MNI space at 2mm voxel resolution, and spatially smoothing using a 6 mm FWHM Gaussian kernel). Z-score maps for the motor tasks were obtained from the general linear model (GLM) analysis using standard SPM canonical HRF (reflecting motor activation vs. rest). Pre-processed rsfMRI data were analyzed using the REST(version 1.8)6 toolkit with de-trending for removal of systematic linear trend. fALFF maps were calculated from de-trended rsfMRI data. Low frequency (0.01-0.08 Hz) bandpass filtering was performed before calculation of a Pearson linear correlation seed based functional connectivity (SCA) map2 & ALFF map from rsfMRI data. For ROI analysis, pre- and post- central gyri were automatically parcellated using an Automated Anatomical Labeling (AAL) template7,8 for each patient. CL (contralesional) and IL (ipsilesional) ROIs circumscribing the combination of pre- and post- central gyri (CG) were obtained for each slice. Consecutive axial sections were evaluated along the z-axis including total extent of pre- & post- CG. Identical ROIs were used for analysis of four maps (tbfMRI, SCA, ALFF, and fALFF).Results
Voxel values in the contralesional (CL) & ipsilesional (IL) ROIs of each map were divided by the corresponding global mean of ALFF & fALFF in the cortical brain tissue. Group analysis revealed significantly decreased IL ALFF (p=0.02) and fALFF (p=0.03) metrics (based on mean of nonzero value voxels) compared to CL ROIs, consistent with similar findings of statistically significantly decreased ipsilesional BOLD signal for tbfMRI (p=0.0005) & SCA maps (p=0.0004). Results for a single subject are shown in Figure 1.Discussion
In this preliminary study we have shown that regional alterations in the frequency domain rsfMRI metrics ALFF & fALFF correspond to similar ipsilesional abnormal activation reductions on tbfMRI & decreased resting state functional connectivity using SCA in the setting of tumor-induced NVU. The abnormally reduced ipsilesional task-based activation in the absence of corresponding neurological deficits or impaired task performance is direct evidence of NVU, whereas the findings on the other maps are resting state correlates of such NVU.Conclusion
The frequency domain metrics ALFF & fALFF may be markers of lesion-induced NVU in rsfMRI similar to previously reported alterations in tbfMRI activation & SCA-derived functional connectivity rsfMRI maps. The potential advantages of the frequency domain metrics include possible absence of network specificity in the assessment of NVU, but this remains to be determined in the future.Acknowledgements
This work is partially supported by NIH grant R42 CA173976-02 (NCI).References
1. Attwell D, et al. Nature 2010;468:232-243
2. Agarwal S, et al. J Magn Reson Imaging 2016 Mar; 43(3):620-6
3. Zacà D, et al. J Magn Reson Imaging 2014;40(2):383-90
4. Zang YF, et al. Brain Dev 2007;29(2):83-91
5. Zou QH, et al., J Neurosci Methods 2008;172(1):137-41
6. Song X-W, et al. PLoS ONE 2011;6(9):e25031
7. Tzourio-Mazoyer N, et al. Neuroimage 2002;15:273–89
8. Smith SM. Hum Brain Mapp 2002;17:143–55
Figures