5315
Functional MRI of Brain's White Matter in Alzheimer's Disease1Health Sciences and Innovation, Fraser Health Authority, Surry, BC, Canada, 2Simon Fraser University, Burnaby, BC, Canada, 3Medical Imaging, Tianjin Medical University General Hospital, Tianjin, People's Republic of China
Synopsis
Impaired white matter (WM) integrity is common in Alzheimer's disease (AD), in addition to gray matter degeneration. While fMRI has been widely used in understanding the disease-associated changes, results so far have omitted the WM even though WM activation has repeatedly been reported in healthy younger adults in recent fMRI studies. Here we applied three tasks targeting interhemispheric transfer at 3.0T to extend the WM fMRI research to clinical applications in AD-dementia. The study detected fMRI activation in the corpus callosal WM in 87% individuals with early AD and normal cognitive aging (NC), and a difference between AD and NC.
Purpose
1) To detect functional MRI activation in brain's white matter (WM) for individuals with early Alzheimer's disease (AD) and normal cognition (NC).
2) To investigate potential differences in the corpus callosal WM fMRI activation between subjects with AD and NC.
Methods
Subjects: Thirty-five older adults participated in the study, including patients who were newly diagnosed as having probable or possible AD (n=10; age=74.2±4.3; women=40%; Table 1) and cognitively normal (n=25; age=70.4±5.2; women=44%; Table 1). Participants were screened to exclude any neurological and psychological conditions other than the target ones.
MRI data acquisition: Imaging data were acquired using a 3.0T whole body system (SignaHDx, General Electric), equipped with an 8-channel head coil. Blood oxygen level dependent (BOLD) fMRI data were acquired using a GRE-EPI sequence (TR=2000ms, TE=30ms, flip angle=90°). Total FOV=224mm×224mm×153mm; 34 axial slices covering the entire brain (matrix=64x64; thickness=4mm, gap=0.5mm). A high-resolution whole brain anatomical T1-weighted imaging was acquired for co-registration (3D-BRAVO; TR/TE=3000ms/min-full, flip angel=13°). FOV=270mm×270 mm, matrix=256×256, with 195 contiguous 1mm slices.
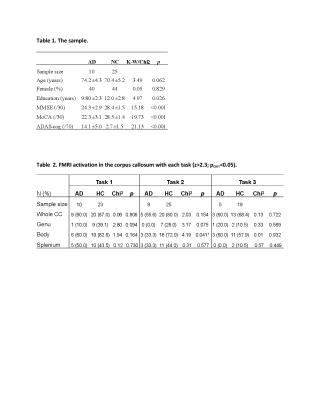
Experiments: During the fMRI session, participants performed three tasks (Figure 1), which targeted the detection of corpus callosal WM activity during information processing and involved lateralized stimulus presentation to elicit inter-hemispheric transfer across the corpus callosum.1-3 The tasks used a block design and were performed in a counter-balanced order by the participants (Figure 1).
Data analysis: Standard pre-processing including motion correction, high-pass filtering (100Hz), spatial smoothing (5mm) used FMRIB Software Library 5.0.9.4 Whole brain activation was examined using a general linear model fitted with a double-gamma hemodynamic response function to predict brain activities in response to the task onset and duration. Individual functional data were registered to structural images and MNI152 space using FLIRT and FNIRT5 for the group mean and the between-group contrast maps. Activation threshold was set at Z>2.3 (p=0.05, cluster corrected).
Results
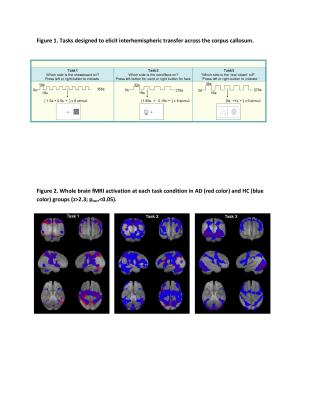
Widespread whole brain fMRI activations were observed in both the NC and AD groups (Figure 2). The activation topography and extent differed across tasks (p<0.05; Figure 2).
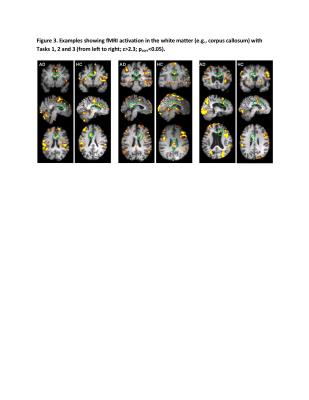
Activations in the corpus callosum were seen in the majority of NC (90%) and AD (87%) individuals (Table 2, Figure 3). WM activation clusters differed by task: while all tasks tended to involve the corpus callosum body, the first two tasks also frequently activated the splenium of corpus callosum.
AD patients showed decreased activation across both the genu and the body of the corpus callosum (30% vs. 72%, p<0.01), especially under Task 2, No participant with AD showed activation in the genu of corpus callosum with any task.
Discussion
The results suggested: 1) the whole brain GM fMRI activation was quite consistent with previous reports, as was the difference between AD and normal aging in the GM;6-8 2) Corpus callosum activation was commonly found in both AD and NC individuals (87%), as previously suggested for healthy younger adults; 1-2,9-12 and 3) there were less WM clusters in AD relative to NC, particularly in the genus of the corpus callosum. The study provided the initial results demonstrating functional WM impairment in AD using fMRI.
Deterioration of WM integrity, in addition to GM atrophy, has been widely reported for people with AD or those at high risk, associated with chronic ischemia and amyloid accumulation and characterized by multiple neuropathological changes.13-15 Many of these changes can alter the task related oxygen energy demands, on which BOLD fMRI signal is based and thus detectable using fMRI.16 Our findings support the hypothesis and suggest that further AD fMRI studies should take WM activation into account.
It is worth noting that fMRI has traditionally excluded the WM, as lower perfusion and scarcity of synapses have caused researchers to discount the possibility of detecting a BOLD response in the WM.16-18 With increases in both signal-to-noise and contrast-to-noise ratio in fMRI, WM activation has been increasingly noted, 2,9-12 although these investigations have been restricted to healthy younger people. The current study contributes to the growing WM fMRI research by demonstrating multiple tasks eliciting interhemispheric transfer across corpus callosum in WM fMRI in aging and AD conditions.
Conclusion
Our data provide the first report of WM fMRI in older adults and the initial demonstration of functional impairment of WM integrity in individuals with AD, implying a new avenue to understanding WM changes in AD-dementia. Ongoing studies by our group are testing these preliminary findings at the group level and examining data within several key WM ROIs in a larger sample size.
Acknowledgements
This research was partially supported by grants from the Canadian Institutes of Health Research (CCI-109608 and CSE125739), the National Science Foundation of China (81010072 and 81401379), and the Fraser Health Research Foundation (FHSPG2015-030). The authors acknowledge Drs. Z. Sun, J. Zhang, S. Beyea and C. Bowen for critical discussions abo the study, and Drs. P. Zhang, X. Lang, and N. Zhang for help with data acquisition and clinical assessments.References
1. Mazerolle EL, D’Arcy RCN, Beyea SD. Detecting functional magnetic resonance imaging activation in white matter: interhemispheric transfer across the corpus callosum. BMC Neurosci. 2008;9,84.
2. Gawryluk JR, D'Arcy RCN, Mazerolle EL, et al. Functional mapping in the corpus callosum: a 4T fMRI study of white matter. Neuroimage. 2011;54:10–15.
3. Song, X, D'Arcy RCN, Fisk J, et al. Correlations of prefrontal cortical activation and cognitive performance with therapeutic cholinesterase inhibitor treatment: a high-field functional magnetic resonance imaging study. Alzheimers Dement. 2011;7, S377.
4. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790.
5. Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Med Imaging Anal. 2001;5:143–156.
6. Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–506.
7. Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50.
8. Li HJ, Hou XH, Liu HH, Yue CL, He Y, Zuo XN. Toward systems neuroscience in mild cognitive impairment and Alzheimer's disease: a meta-analysis of 75 fMRI studies. Hum Brain Mapp. 2015;36:1217–1232.
9. Tettamanti M, Paulesu E, Scifo P, et al. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol. 2002;88:1051–1058.
10. D'Arcy RC, Hamilton A, Jarmasz M, et al. Exploratory data analysis reveals visuovisual interhemispheric transfer in functional magnetic resonance imaging. Magn Reson Med. 2006;55:952-958.
11. Gawryluk, J. R., D’Arcy, R. C. N., Mazerolle, E. L., Brewer, K. D., and Beyea, S. D. (2011). Functional mapping in the corpus callosum: a 4T fMRI study of white matter. Neuroimage. 54, 10–15.
12. Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T. Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res. 2011;1370:99–111.
13. Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262.
14. Bartzokis G, Cummings JL, Sultzer D. White matter structural integrity in healthy aging adults and patients with Alzheimer’s disease: A magnetic resonance imaging study. Arch Neurol 2003;60:393–398.
15. Rieckmann A, Van Dijk KRA, Sperling RA, et al. Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer's disease. Neurobiol Aging. 2016;177–88.
16. Gawryluk, JR, Mazerolle EL, D’Arcy RCN. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci. 2014;8,239.
17. Logothetis NK and Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66: 735–769.
18. Rostrup E, Law I, Blinkenberg M, et al. Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. Neuroimage. 2002;11:87–97.