5314
fMRI activation optimization in the setting of brain tumor-induced neurovascular uncoupling using resting state BOLD ALFF1Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
The phenomenon of neurovascular uncoupling (NVU) is an under-recognized but very important limitation of clinical BOLD fMRI because it can lead to non-visualization of eloquent cortex and resultant inadvertent surgical resection of vital brain tissue leading to permanent postoperative disability. In this study we demonstrate a novel method for correcting for the spuriously decreased ipsilesional motor activation associated with NVU through use of a novel resting state fMRI (rsfMRI) frequency domain metric-- ALFF (amplitude of low-frequency fluctuation)-- in patients with perirolandic low grade gliomas.
Purpose
Blood Oxygen Level Dependent functional magnetic resonance imaging (BOLD fMRI) is an indirect measure of neuronal activity. BOLD fMRI detects alterations in deoxyhemoglobin concentration influenced by hemodynamic factors that occur in response to neuronal activity. The coupling between neuronal activity and the hemodynamic changes occurring in the adjacent vasculature is often disrupted in patients with brain tumors, resulting in abnormally decreased ipsilesional BOLD fMRI activation1. In this study we propose a novel method of correcting for the effects of NVU on task-based fMRI (tbfMRI) activation using the resting state BOLD frequency domain metric ALFF (amplitude of low-frequency fluctuation2). We will apply this method specifically to correct for NVU affecting the primary motor cortex.Methods
Twelve de novo brain tumor patients who underwent clinical fMRI exams including tbfMRI and resting state fMRI (rsfMRI) on a 3T MRI system were included in this IRB-approved study. Each patient displayed decreased/absent tbfMRI activation in the primary ipsilesional sensorimotor cortex in the absence of corresponding motor deficit or suboptimal task performance, consistent with NVU.1 Imaging was performed on a 3.0 T Siemens Trio MRI with a 12-channel head matrix coil using a 3D T1 MPRAGE (TR=2300 ms, TI= 900 ms, TE= 3.5 ms, 9° FA, 24-cm FOV, 256x 256x176 matrix, slice thickness 1 mm) for structural imaging and multiple 2D GE-EPI T2* weighted BOLD sequences for both task & resting functional imaging (TR=2000 ms, TE=30 ms, 90° FA, 24-cm FOV, 64x64x33 matrix, 4 mm slice thickness with 1 mm gap between slices, interleaved acquisition). 180 volumes were acquired in 6 minute long rsfMRI scan. A vertical tongue movement task and a bilateral simultaneous sequential finger tapping task (each 3 minutes duration with alternating 30 second blocks of movement and rest) were used for tbfMRI. Instructions for all tasks were visually cued. SPM12 was used for preprocessing of tbfMRI & rsfMRI data (slice timing correction, realignment, normalization to MNI space at 2mm voxel resolution, and spatially smoothing using a 6 mm FWHM Gaussian kernel). Z-score maps for the motor tasks were obtained from the general linear model (GLM) analysis using standard SPM canonical HRF (reflecting motor activation vs. rest). Pre-processed rsfMRI data were analyzed using the REST(version 1.8)3 toolkit. After de-trending for removal of systematic linear trend and low frequency (0.01-0.08 Hz) bandpass filtering ALFF maps were calculated from rsfMRI data. Pre- and post- central gyri were automatically parcellated using an Automated Anatomical Labeling (AAL) template4,5 for each patient. CL (contralesional) and IL (ipsilesional) ROIs circumscribing the combination of pre- and post- central gyri (CG) were obtained for each slice. Consecutive axial sections were evaluated along the z-axis along the craniocaudal length of the tumors . Identical ROIs were used for analysis of both maps (tbfMRI & ALFF). Mean of ALFF (mALFF) in CL ROI is calculated. Motor activation maps were further analyzed using Amplitude Measured as a Percentage of Local Excitation (AMPLE) thresholding of 50% (i.e., only voxels with Z scores above 50% of a local cluster Z score maximum were considered “active”)6. Ipsilesional voxels with sub-threshold activation (between 25% to 50% of a local cluster Z score maximum) in motor task activation map were considered to have falsely reduced activation due to NVU and were subsequently corrected by enhancing their voxel Z-score values by a factor representing the quotient obtained by dividing the contralesional mALFF by the individual ipsilesional voxel ALFF value. For each patient, motor activation maps pre- and post ALFF correction were obtained and their difference was evaluated.Results
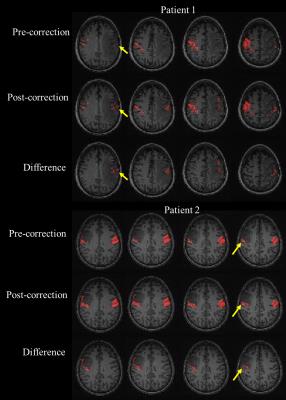
Motor activation maps for two tumor patients as pre- ALFF correction, post- ALFF correction, as well as their difference map, obtained by subtracting the pre-correction map from the post-correction map, are depicted in Figure 1. The number of voxels in the ipsilesional (IL) ROI for each subject pre- and post- ALFF correction was obtained and a group analysis was performed which reveals significantly increased number of voxels displaying ipsilesional motor cortical activation post-correction compared to pre-ALFF correction (p=0.00002 using paired t-test) .Discussion
In this preliminary study we demonstrate the feasibility of optimization of primary motor cortical activation in the setting of perirolandic tumor-induced NVU through use of a novel resting state BOLD ALFF-based correction algorithm.Conclusion
This study is the first to demonstrate successful application of a novel resting state ALFF-based correction algorithm for optimization of motor cortical activation in cases of tumor-related neurovascular uncoupling.Acknowledgements
This work is partially supported by NIH grant R42 CA173976-02 (NCI).References
1. Zacà D, et al. J Magn Reson Imaging 2014;40(2):383-90
2. Zang YF, et al. Brain Dev 2007;29(2):83-91
3. Song X-W, et al. PLoS ONE 2011;6(9):e25031
4. Tzourio-Mazoyer N, et al. Neuroimage 2002;15:273–89
5. Smith SM. Hum Brain Mapp 2002;17:143–55
6. Voyvodic et al.,JMRI 2009; 29(4):751-9.
Figures