5310
Pharmacological inactivation of dorsal hippocampus enhances responses and induces adaptation to sound in midbrain1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, People's Republic of China, 4Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, Hong Kong
Synopsis
The hippocampus is associated with the memory and learning, meanwhile, receives
Purpose
The dorsal hippocampus receives auditory input from the central auditory system by continuous communication with the neocortex via the hippocampal-neocortical network1,2. Recent resting-state fMRI study showed that low frequency optogenetic stimulation of the dorsal hippocampus enhances brain functional connectivity, including the primary auditory network3. However, whether and how the dorsal hippocampus contributes to sound processing in auditory system remains unknown. The auditory midbrain, the inferior colliculus (IC), is a pivotal station of the auditory system, integrating the information from various auditory sources and cortical feedback4. Tetrodotoxin (TTX) blocks the voltage-dependent sodium channels to inactivate neuronal activity5. In this study, we investigate the influence of dorsal hippocampus on sound processing in the IC by combing pharmacological inactivation and auditory fMRI.Methods
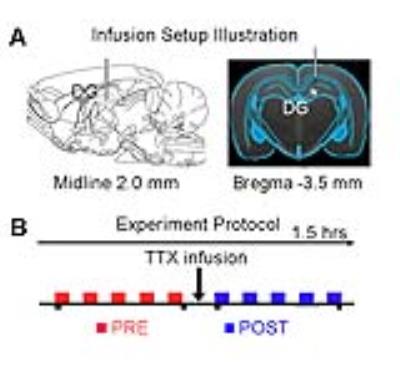
Adult Sprague-Dawley rats (n=7, 11 weeks old, male) were stereotaxically implanted an MRI-compatible cannula (internal diameter: 250μm) in the right dorsal dentate gyrus (dDG), to infuse TTX during fMRI experiments (Figure 1A). Totally ten auditory fMRI sessions were performed on each animal. After five sessions, 5μL TTX (concentration: 5-10ng/μL) was injected into dDG (Figure 1B). During the experiment, animals were ventilated and anesthetized with 1.0% isoflurane. Monaural (left) broadband noise stimulation (sound pressure level = 85dB) was presented in a block design paradigm (20s on and 40s off, 4 blocks) via a custom-made 165cm long tube6. All fMRI data was acquired on a 7T Bruker scanner using GE-EPI (FOV=32×32mm, matrix=64×64, α=56°, TE/TR=20/1000ms, sixteen 1.0mm slices without gap). Data were first realigned, co-registered, in-plane smoothed and high-pass filtered before the standard GLM analysis was applied to identify significant BOLD responses.Results
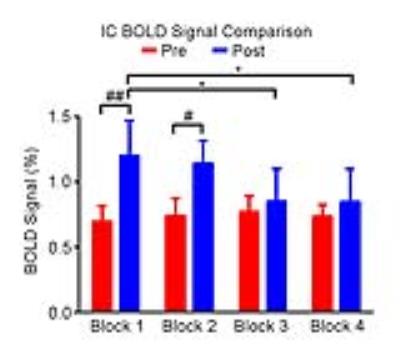
Figure 2 shows the responses in the inferior colliculus (IC) to each block of noise stimulation before and after TTX infusion. Figure 3 presents the comparisons of averaged BOLD signal percentage change. A significant increase was observed after TTX infusion in the first two blocks. Note that there were no apparent differences in the response in the IC among blocks before TTX infusion, whereas the amplitude of BOLD signal was decreased block by block after infusion, indicating an adaptation to noise.
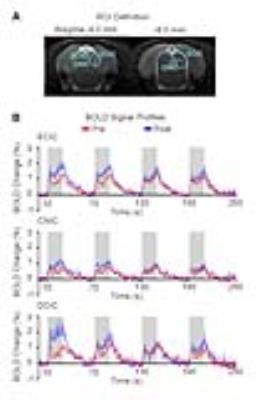
Figure 4 presents the responses in different subregions of IC, including external cortex of the IC (ECIC), central nucleus of the IC (CNIC) and dorsal cortex of the IC (DCIC). The difference in response of the first block before and after TTX infusion was largest in DCIC, followed by ECIC and CNIC.
Discussion and Conclusion
Firstly, our results showed apparent response increase within the first block during pharmacological TTX inactivation of the dorsal hippocampus. A recent rsfMRI study reported that activating the dorsal hippocampus increased the interhemispheric connectivity in auditory cortex, suggesting that hippocampal input may enhance intrinsic activity in the AC3. In addition, an AC ablation fMRI study showed that IC response was inhibited by AC via descending projections6. Together, these two studies suggest that lack of hippocampal inputs may lead to a decrease in intrinsic activity in AC, and consequently a decreased inhibition effect from AC to IC, i.e., IC responses could be stronger upon auditory stimulation, which was in agreement with our present findings. Thus this enhancement effect likely occurred through the hippocampal-cortical and the corticofugal pathway to the IC. Secondly, our results revealed that inactivation of hippocampus induces the adaptation to noise in the IC, suggesting that the hippocampus contributes to the gain modulation of IC responses. Other studies also indicated that the hippocampus plays a role for temporal processing of sensory information7 and novelty detection8 in the IC. Our results here further supported that the hippocampus exhibits a functional and dynamic effect on temporal auditory processing in the IC. Furthermore, inactivation of the hippocampus induced different trends in three subregions of the IC, suggesting that hippocampus mediates various aspects of auditory processing in the IC. The adaptation observed in DCIC was the most apparent likely because DCIC is regarded as mainly under the influence of the descending pathway9. CNIC exhibited least influence by inactivation of hippocampus, likely because CNIC mainly receive massive ascending input from lower auditory regions but not descending input or commissural input10. ECIC also showed adaptation, which may occur by feedback signal from cortex and intrinsic connections among subregions of the IC9. In summary, our present auditory fMRI study demonstrated the influence of the hippocampus on temporal sound processing in the IC, with an initial increase in the first block and followed by adaptation when the hippocampus is pharmacologically inactivated. Our fMRI findings present the first and direct experimental evidence that the hippocampus plays a dynamic role in shaping the central auditory processing at midbrain.Acknowledgements
No acknowledgement found.References
1. P. Lavenex and D. G. Amaral, "Hippocampal-neocortical interaction: a hierarchy of associativity," Hippocampus, vol. 10, pp. 420-30, 2000.
2. K. S. Kraus and B. Canlon, "Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus," Hearing research, vol. 288, pp. 34-46, 2012.
3. R. W. Chan, A. T. L. Leong, P. P. Gao, Y. S. Chan, W. H. Yung, K. K. Tsia, et al., "Low Frequency Optogenetic Stimulation of Dentate Gyrus Enhances Brain Functional Connectivity Revealed by Resting-State fMRI," ISMRM, p. 0311, 2016.
4. J. H. Casseday, T. Fremouw, and E. Covey, "The inferior colliculus: a hub for the central auditory system," in Integrative functions in the mammalian auditory pathway, ed: Springer, 2002, pp. 238-318.
5. M. Kaneda, Y. Oyama, Y. Ikemoto, and N. Akaike, "Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine," Brain Res, vol. 484, pp. 348-51, Apr 10 1989.
6. P. P. Gao, J. W. Zhang, S.-J. Fan, D. H. Sanes, and E. X. Wu, "Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study," NeuroImage, vol. 123, pp. 22-32, 2015.
7. E. Nyhus and T. Curran, "Functional role of gamma and theta oscillations in episodic memory," Neuroscience & Biobehavioral Reviews, vol. 34, pp. 1023-1035, 2010.
8. T. Liberman, R. A. Velluti, and M. Pedemonte, "Temporal correlation between auditory neurons and the hippocampal theta rhythm induced by novel stimulations in awake guinea pigs," Brain research, vol. 1298, pp. 70-77, 2009.
9. G. Paxinos, The Rat Nervous System: Gulf Professional Publishing, 2004.
10. G. Celesia and G. Hickok, "Auditory pathways: anatomy and physiology," The Human Auditory System: Fundamental Organization and Clinical Disorders, vol. 129, p. 3, 2015.
Figures